Last December, Sanford Simon attended a cell biology meeting where researchers presented picture after picture of cells colorfully highlighted by organic dyes or fluorescent proteins. Speakers also debuted movies–featuring proteins as cellular action heroes. In these little dramas, often lasting only seconds, viewers witnessed the complicated molecular actions underlying cancer, diabetes, and other human diseases.
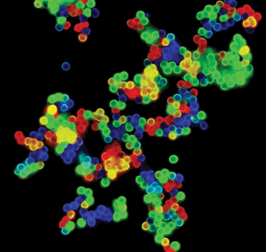
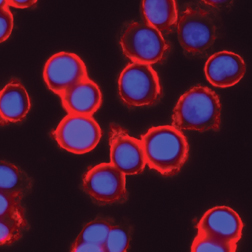
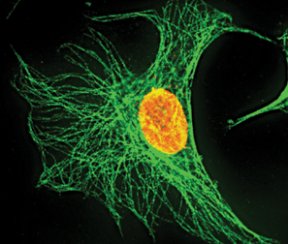

Such colorful demonstrations pervade biology research, says Simon, a biologist at the Rockefeller University in New York, where he does plenty of cellular photography of his own. Pick up almost any molecular biology journal and there’s a gorgeous cell on its cover, glowing brightly in green or red or an entire rainbow of colors. “Part of this [imaging] is intellectual curiosity and part of it is a real hope of understanding more about human physiology and pathology,” says Simon.
But the widely used dyes and proteins, called fluorophores, have drawbacks. They fade quickly, and only two or three colors can be used simultaneously to label different cellular components. Biologists would prefer to see all the machinery of a cell operating at once in Technicolor over days, months, or even years.
That’s where quantum dots come into the picture. Most often touted for their potential roles in computing and data storage, these nanoscale particles of semiconductor can be made to fluoresce in any color for months and perhaps years. In 1998, two landmark publications heralded quantum dots as labels for cells and proteins. “It seemed they had the potential for solving all sorts of problems we were having with imaging in biology,” says Simon, who has done fluorescent imaging for years.
Now, 4 years later, a landslide of new research is showing that the initial enthusiasm for dots in biology wasn’t simply hype. They just might be the next stars of biological imaging.
A better beacon
Shuming Nie images cells primarily to determine whether a tissue sample contains markers of a particular cancer. Quantum dots could provide faster, more sensitive, and maybe cheaper imaging than conventional fluorophores do, says Nie, a researcher at the Georgia Institute of Technology and director of cancer nanotechnology for the Winship Cancer Institute at Emory University in Atlanta. Eventually, sensitive, long-duration imaging might even reveal precisely how wayward proteins or cells–such as those involved in Alzheimer’s disease or cancer–behave in the body.
Says Nie: “I think this quantum-dot thing is going to be the first example of nanotechnology that can really have some practical applications.”
Four years ago, while working at Indiana University in Bloomington, Nie and Warren C.W. Chan published one of the two simultaneous reports that showed how semiconductor quantum dots could tag cells with microscopic beacons of light. The other report was by A. Paul Alivisatos and his colleagues at the University of California, Berkeley.
The semiconductor particles that researchers are grooming for biological imaging are generally made of a cadmium selenide core surrounded by a shell of zinc sulfide.
Their diameters measure in nanometers. When hit with light, the quantum dot emits a particular color based on its size. Smaller dots fluoresce at shorter wavelengths, such as blue, while larger dots emit longer wavelengths, like red.
Quantum dots have significant advantages over earlier techniques. Typically, researchers can view no more than three colors at once with ordinary fluorescence labeling using proteins, such as green fluorescent protein, or organic dyes, such as rhodamine. Each of the fluorophores must first be excited with a specific wavelength of light, which can block the emitted color of a second or third fluorescent tag. To overcome this problem, researchers can mark multiple proteins in a cell with several different colors, photograph them at different times, and then superimpose the pictures. Or researchers can tag one protein in one cell and another in a similar cell.
Simon likens the problem to that of watching his kids play games of Monopoly on consecutive days, but only seeing his daughter’s first turn on the first day, his son’s second turn on the second day, and so on. “What you want to do is see both of them playing at the same time in the same game,” he says.
With quantum dots, scientists can simultaneously view many different markers in the same cell. All quantum dots light up when hit with the same wavelength of light, no matter how many different dot sizes–and therefore colors–are in a sample.
The dots’ colors are also very specific, so they don’t generally overlap one another, and many different colors can be used at once.
To create an even larger palette of colored labels, Nie recently embedded hundreds of dots in a single micronwide polymer bead. Each of Nie’s beads is chemically linked to an antibody that can home in on and stick to a particular protein, and the intensity of a bead’s emission increases proportionately with the number of dots it carries. A year-old company in Pittsburgh called Bioplex Corp. aims to commercialize these beads, says Nie, a consultant to the company. This approach could give biological imaging a huge pool of quantum-dot labels, he says.
Another limitation of conventional fluorophores is their short life span. They can fade in a couple of hours. In contrast, quantum dots remain stable for days to months. Potentially, a tissue sample with quantum-dot labels could be archived for years, says Nie.
In 1998, when Nie and Alivisatos first floated the idea of using quantum dots for studying cells, the technology was still young and rife with problems. For instance, says Xingyong Wu of Quantum Dot Corp. in Hayward, Calif., the dots weren’t picky enough about which cellular proteins or structures they tagged, and researchers needed better ways to make the dots water soluble and to link them to antibodies and other molecules. And if those challenges could be met, would quantum dots be useful?
They might disrupt or kill cells. After all, one of their major ingredients, cadmium, is extremely toxic.
Bio dots
In the past 4 years, scientists have worked out enough of the kinks in quantum-dot technology to make it ready for roles in biological imaging, says Nie. A trio of new studies has shown that researchers can chemically link an antibody or a specialized molecule to a dot so that it binds only to specific proteins in a cell or a tissue sample. Scientists have also learned to inject dots directly into a cell or permit cells to engulf the particles. Then, researchers can track the cell through an organism’s development.
“These three papers clearly represent a milestone in the biological applications” of quantum dots, says Nie. “Now, the game is open.”
These papers relied on recent advances in quantum-dot surface chemistry. For instance, the dots themselves are hydrophobic, so they resist dissolving in the water that dominates living tissue. Although Nie’s and Alivisatos’ groups each found a way to make their dots water-soluble, researchers have been striving to invent new methods that are quicker and easier and end up with particles that fluoresce longer and are less likely to poison samples.
What’s more, the dots’ surfaces need a versatile way to link with a variety of antibodies and other molecules that will target specific cell structures. New coatings include silicon shells, polymer spheres, and self-assembling molecules that tightly pack themselves over a dot.
Instead of coating or encapsulating dots, Hedi Mattoussi of the U.S. Naval Research Laboratory (NRL) in Washington, D.C., and his colleagues chemically replace the water-hating surface of a dot with a new surface that’s more tolerant of water. These dots maintain their capacity to fluoresce for more than a year, even when kept in water, says Mattoussi, but he’d like to make dots that are stable forever.
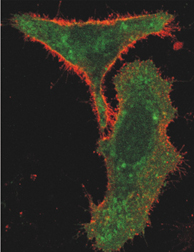
In one of the three recent reports, Mattoussi and his NRL coworkers, collaborating with Simon’s research group at Rockefeller, address another problem with quantum dots: their specificity. In the January Nature Biotechnology, the researchers show that quantum dots can label cellular proteins as specifically as does one of today’s gold standard fluorophores–green fluorescent protein, first found in certain jellyfish. Simon’s team engineered mammalian cells to produce proteins on their surfaces that have green fluorescent protein attached to them and are markers for resistance to certain therapeutic drugs.
In one experiment, the scientists exposed cells to antibodies that bind to the drug-resistance protein. Then, they added orange-emitting quantum dots that they had previously linked to avidin, a molecule that readily binds to these antibodies. In another experiment, the team chemically attached the antibody to the quantum dots by way of a linker molecule, protein G, and then put them in dishes with cells. In both experiments, the researchers observed that orange fluorescence from the quantum dots precisely matched the pattern of fluorescence from green fluorescence protein attached to the cell-surface proteins.
To label specific proteins not just on cell surfaces but also in a cell’s cytoplasm and nucleus, Wu and his coworkers at Quantum Dot and at Genentech in South San Francisco, Calif., used techniques similar to those of Simon’s group. They linked the molecules streptavidin or immunoglobulin G to their dots. With their quantum dots in tow, these molecules then sought out antibodies clinging to proteins on cells. This technique enabled Wu’s group to detect and label breast cancer markers on the outside surfaces of cells living in lab dishes.
To investigate the inside of cells, the researchers introduced streptavidin-linked quantum dots into the cytoplasm of specially prepared, dead cells. The dots labeled the actin filaments and microtubule fibers that make up the cell skeleton. They also tagged proteins within the nucleus. Whether on the surface or inside the cells, the quantum-dot beacons are brighter and more stable than conventional organic dyes, the researchers note in the January Nature Biotechnology.
But what about labeling the inside of living cells? To address that question, Benoit Dubertret of the Laboratoire d’Optique Physique at CNRS in Paris and his colleagues at three U.S. institutions injected solutions of quantum dots into frog embryos and watched the organisms grow for 5 days. The researchers found that when cells divided, quantum dots ended up in the daughter cells. This finding, published in the Nov. 29, 2002 Science, enabled the scientists to follow the organisms’ development.
Aside from answering basic biological questions about embryo development, this labeling indicates that quantum dots are not toxic to these cells, says Dubertret. If the dots had been toxic, he says, the embryos probably would have become malformed.
Simon’s group explored toxicity by testing its quantum dots in both human and slime mold cells. In these cases, the researchers let the cells engulf quantum dots through a natural cellular process called endocytosis. Neither cell type showed any obvious problem, the team reports in its Nature Biotechnology paper.
What’s more, these results led Simon and his colleagues to investigate an age-old, baffling question about slime mold behavior. When these single-celled organisms are starving, they send out chemical signals that encourage their neighbors to join them in a large multicellular mass. But researchers haven’t known whether this is an all-or-nothing response to a nutrient deficiency or a response proportional to how hungry the cells are.
Long-lasting quantum-dot labeling gave the researchers a tool to find out. While starving cells for various lengths of time, Simon and his colleagues labeled them with quantum dots. They found that starved cells were just as likely to aggregate no matter how many hours they’d been without nutrients. As expected, cells that hadn’t been starved didn’t aggregate.
Beyond such basic science, the tags are also now ready for use in laboratory diagnostic tests, Nie suggests. He says that his own lab has demonstrated that quantum dots could effectively label cancer markers in tissues removed from patients.
Since certain drugs are effective only for patients with particular marker proteins, doctors can use such information to tailor treatments for particular patients.
Ultimately, scientists might track a cancer cell that moves through the body. But such internal applications of quantum dots will require that rigorous toxicity studies show no harm to patients.
Quantum dots are already poised to join traditional fluorophores as a tool important to biologists. The hundreds of fluorescent images at the cell biology meeting last December indicate that biological labeling has “just exploded,” says Simon. “It’s both exciting and humbling to see the creative ways scientists are now applying [green fluorescent protein],” he says. “I think you’re going to see the same thing now with the quantum dots as well.”
****************
If you have a comment on this article that you would like considered for publication in Science News, please send it to editors@sciencenews.org.
To subscribe to Science News (print), go to https://www.kable.com/pub/scnw/
subServices.asp.
To sign up for the free weekly e-LETTER from Science News, go to http://www.sciencenews.org/subscribe_form.asp.