This soft, electronic ‘nerve cooler’ could be a new way to relieve pain
The device uses evaporative cooling to block pain signals, experiments in rats suggest
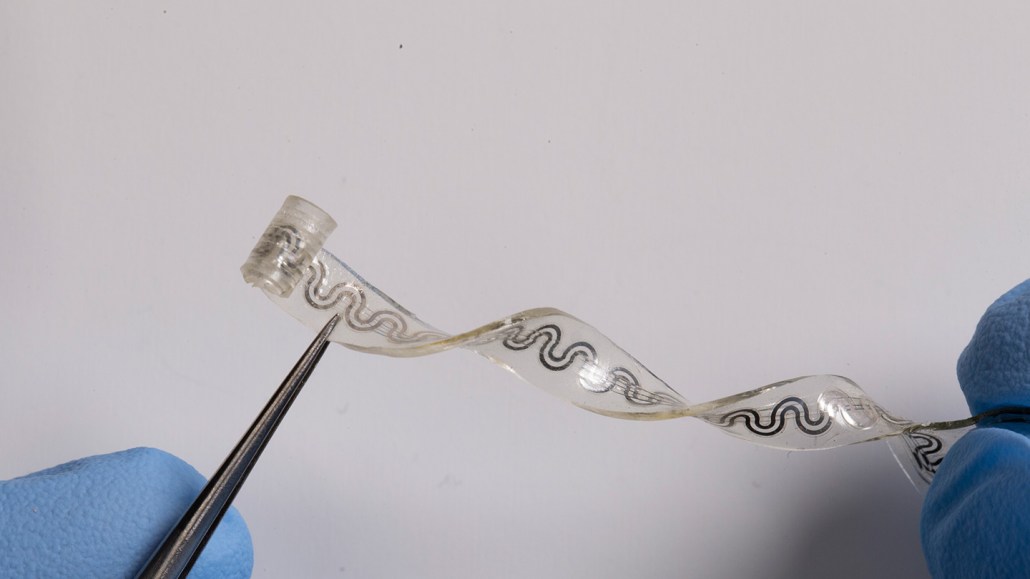
This flexible, dissolvable implant relieves pain by using evaporative cooling on nerves.
Northwestern University







