Pain, numbness, pain
Scientists have pinpointed the trigger that leads to a burning feeling when some anesthetics are administered.
100….98….97….96….95….
“Relax. Keep counting,” the anesthesiologist says to the patient, who is having her hip surgically replaced. “You won’t feel a thing.”
Though the woman can no longer feel the dull ache in her hip, she can feel a prickly burning in her arm where a general anesthetic drips intravenously into her veins. It is a sensation she doesn’t remember the doctor telling her about, she thinks, as she forces herself to mouth a few more numbers…94 ….93….92….
For years patients have reported a burning feeling at the site of a general anesthetic injection, or in the lungs when inhaling gaseous forms of the drugs, which put a patient into an unconscious sleep. But doctors could not pinpoint where the pain response originated — until now.
Science has finally confirmed the patients’ perspectives and identified the pain-promoting trigger associated with anesthesia. What’s more, they find that some anesthetics can also increase pain after surgery by adding to swelling of the tissue being operated on, a team of scientists from Georgetown University in Washington, D.C., report in the June 23 Proceedings of the National Academy of Sciences.
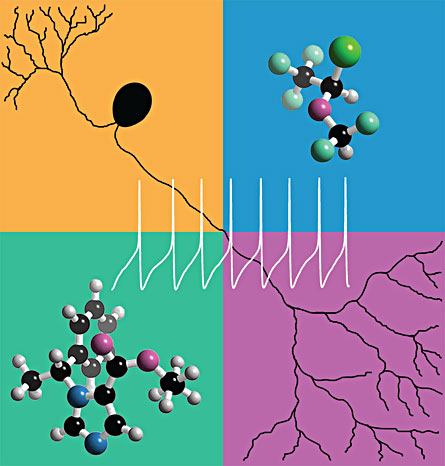
The ion channel protein called TRPA1 is present on sensory neurons, which are located in most body tissues. Anesthetics activate this protein, also called the mustard-oil receptor, causing nerves to “fire.” That message tells the brain that something painful is happening.
“Probably what is most significant for people to know is that this activation of a pain channel actually adds to post-surgery inflammation, so what we didn’t know before was that you could exacerbate swelling of surgery-damaged tissue with general anesthetics,” says Georgetown neuroscientist Gerard Ahern, who oversaw the new study, with lead author and Georgetown postdoctoral researcher José Matta. “I don’t think anyone has ever considered that before.”
Until now, scientists did not understand the mechanism by which the anesthetics activate and sensitize pain-response neurons in the peripheral nervous system. So anesthesiologists have not known how to reduce the drugs’ painful side effects. The results may lead to development of new anesthetics or increased use of the few anesthetics that don’t have these pain-inducing side effects.
“Now that we know the mechanism that triggers these ill side effects … we can get closer to finding a single agent, a single general anesthetic to use rather than a bunch of drugs together,” says Tim Hales, a neuropharmocologist at The George Washington University in Washington, D.C., who was not involved in the research. “The fewer drugs we have to administer, the safer it is for the patient. That single anesthetic is the ultimate, long-term goal.”
Each year, more than a hundred million people go under the knife, according to GeorgetownUniversityMedicalCenter. Doctors have known for a long time that patients experience burning where general anesthetics are injected, Ahern says. That is why anesthesiologists typically give a local anesthetic such as lidocaine to dull that pain. But it does not always stop the burning. Inhaling gases that cause temporary unconsciousness can also cause patients to experience a burning in their lungs, he says.
Still, general anesthetics make modern surgery possible because they block the memory and sensation of pain while a patient is under, says University of Wisconsin–Madison physiologist Mathew Jones, who was not involved in the research. The drugs, he says, are not designed to reduce pain but make a patient unaware of it by affecting the central nervous system — the brain and the spinal cord.
Most general anesthetics have the undesirable side effect of actually causing pain or irritation when first administered, before the patient goes to sleep, because they trigger a response in the peripheral nervous system, an extension of the central nervous system that includes pain receptors. The anesthetics activate TRPA1 on pain-sensing nerves, the new study confirms.
The anesthetics that evoke this sensory response of pain and swelling are called “noxious” or pungent anesthetics. In this case “noxious” means merely that the anesthetic causes a sensory response. It is the chemical structure of the drug that determines whether it will excite the pain-receptor nerve cells.
The new study verifies that the few non-pungent anesthetics that do exist do not activate the pain sensory neurons. But, Ahern says, the majority of general anesthetics used worldwide are pungent.
When the sensory nerve receptors at the injection site are excited, they release intercellular messengers that tell the body to send extra white blood cells, and those cells target tissue damaged during surgery. The white blood cells prevent against infection but also cause swelling and pain.
Ahern explains that a patient will always have pain and swelling after surgery because slicing through the tissue already elicits a white blood cell response. The pungent anesthetic’s activation, however, adds to that white blood cell response, which increases pain and inflammation at the site of the surgical incision when the patient wakes up.
Usually an anesthetic is injected, Ahern explains, “but if surgery lasts beyond five minutes, the anesthetic is maintained with gas anesthetics, which can add to the normal swelling caused after surgical damage to the tissue stops.”
Finding the trigger
Ahern
and researchers in his Georgetown
lab wanted to pinpoint this pain-causing mechanism. They decided they would
first test two specific sensory nerve receptors known to react to other
irritants — specifically wasabi, garlic and capsaicin, the chemical that puts
the bite in hot chili peppers. When the chemicals activate the sensory nerve
receptors, humans feel a burning sensation, Ahern says, and “it seemed that the
receptors reacted to pungent anesthetics in the same way.”
By inserting DNA that codes for the two kinds of sensory neuron proteins into human embryonic kidney cells, which do not normally have this sensory-coding genetic material, the team isolated the sensory receptors in a Petri dish. They watched the specific mechanisms by which the receptors responded to the different anesthetics.
The most commonly used, pungent anesthetics, such as the intravenous liquid propofol and the gas isoflurane, activated a response only from TRPA1. It is a principal receptor in the pain pathway. The tests clearly showed that TRPA1 is activated selectively by pungent anesthetics and not by the non-pungent anesthetics, Matta says.
But the scientists wanted to make sure that TRPA1 was the same receptor that caused pain in living animals and possibly humans. To see that it was, the researchers applied the anesthesia propofol to the inner nose tissue of a group of normal mice and a group of mice lacking TRPA1 receptors. The normal mice felt the burn and tried to rub off the anesthetic by furiously wiping their noses in their cage sawdust. The mutant mice did not react.
To study whether the TRPA1 receptor also triggered additional inflammation as it would after surgery, “we tried to mimic a human operation,” Ahern says. The scientists induced what would be “post-operative swelling” by rubbing mustard oil onto one ear of the mice. TRPA1, the mustard-oil receptor, naturally reacted to the oil, causing the mice’s affected ears to puff up. The researchers measured the enlargement in comparison to the unaffected ear and then anesthetized the mice with either isoflurane or another anesthetic, called sevoflurane — a non-pungent anesthetic. Measuring the changes in swelling showed that isoflurane, the more commonly used anesthetic, added to ear inflammation. Sevoflurane did not.
Easing the pain
“This is an elegant study because of the breadth of the testing,” Hales says. “Here we are seeing what happens with specific neuron receptors in a Petri dish and can also see what happens in a whole animal and what the behaviors responses are.”
Jones too was impressed by the detail of the study. “These authors hit the problem at all levels, which allows them to understand the phenomenon from single molecules all the way up to animal behavior,” he says. “That’s cool.”
This work will be cited by others in the area, “including myself,” adds Hales, who studies general anesthetics. Right now, he says, anesthesiologists use an armamentarium of different drugs to put a patient under because one cannot do it well enough. If attention is given to this new work, anesthesiologists will notice it and might start thinking about what is the best drug to use to induce unconsciousness and possibly reduce post-operation swelling and pain, he explains.
But the scientists agree there is still a lot more to choosing an anesthetic than just reducing painful side effects. Some of the non-pungent drugs, like halothane, have other undesirable effects — such as causing cardiac arrhythmias — or are more expensive, which is the case with sevoflurane. So right now, Jones says, the anesthesiologist will still have to decide on a case by case basis, depending on the patient’s condition, history and what other drugs might be on board during the surgery.
By knowing about this pain-causing mechanism, it may now be possible to avoid certain general anesthetics’ side effects either by using TRPA1 blockers along with the drugs or by designing better anesthetics that don’t activate TRPA1 in the first place, both Matta and Jones say.
Although it seems natural to assume that reducing inflammation will be beneficial, scientists have learned that this may not always be the case, says Robert Pearce, who chairs the anesthesiology department at the University of Wisconsin–Madison and was also not involved in the study. Studying the inflammatory effects of the different anesthetics in humans and understanding the healing process will let doctors determine whether the pain of more inflammation is worth it to gain the benefit of healthier tissue healing, Pearce explains.
“At the very least, this provides a first-pass screen for whether an anesthetic is going to be pungent in patients,” Jones says. Knowing whether a general anesthetic does or does not evoke a response “will save a lot of money, time and research animals during the drug development process.”