Putting African sleeping sickness to bed
Mouse experiments could lead to new treatment for parasitic disease
A new drug may lead to a much-needed treatment for African sleeping sickness, a parasitic disease estimated to kill more than 30,000 people worldwide each year. The compound rids mice of the parasite, the protozoan Trypanosoma brucei, scientists report April 1 in Nature.
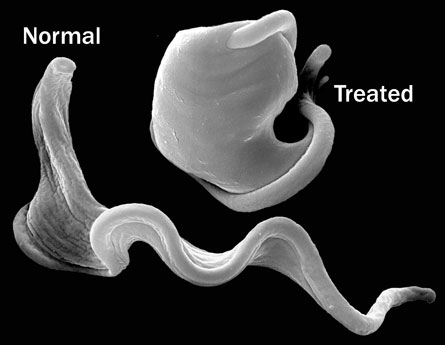
Left untreated, sleeping sickness is almost always fatal, yet the available drugs are considered dangerous and ineffective. The new drug, which can be made cheaply and could be taken orally, kills the microbe by targeting a key protein in T. brucei.
“Having a new drug target is exciting,” says study coauthor Paul G. Wyatt, a medicinal chemist of the University of Dundee in Scotland. “Having a molecule that can affect that target is even more exciting.”
The parasite, delivered by the bite of the tsetse fly, first infects the victim’s bloodstream. After several months (or years, depending on the type of infection), T. brucei then invades the brain, causing disturbed sleeping patterns, dementia and comas, before ultimately killing the infected person. Currently, side effects from one of the most common drugs used to combat the parasite kill about 1 out of 20 people, says biochemist Dietmar Steverding of the University of East Anglia in Norwich, England.
The results are “very promising,” Steverding comments. “They’ve come up with a quite potent drug.”
Wyatt and his colleagues followed a lead from earlier studies that found T. brucei couldn’t grow without a naturally occurring protein called NMT. This protein is an enzyme that affixes chains of fatty acids to other proteins. These fatty acids perform a variety of important roles, including acting as location tags to direct proteins to certain parts of the cell. NMT acts on at least 60 proteins in the cell, so interfering with NMT has “very dramatic effects on the parasite,” Wyatt says.
Screening more than 60,000 small molecules turned up some that disturbed NMT function. Wyatt and his team tweaked the chemistry of these molecules and ended up with one, which they call DDD85646, that stopped the parasites from multiplying in test tube experiments. Oral doses of this compound knocked out the infection in mice with sleeping sickness, the team reports. Further experiments revealed that the drug binds to a small molecular pocket on the NMT protein and prevents the protein from affixing fatty acids to its targets.
The exact sequence of events that kills the parasites is unclear, but the researchers suspect that the drug damages the parasite’s protective outer membrane. To thwart the body’s immune system, the parasite frequently changes the composition of its coating, and Wyatt and his colleagues think that their compound interferes with T. brucei’s ability to renew its membrane. “Normally long, thin, elegant cells turn into fat, bloated round cells,” after they’ve been hit with the drug, Wyatt says.
In the experiments on mice, the compound effectively wiped out the parasite in blood but could not penetrate into the brain, meaning it won’t be effective for the second, more advanced stage of sleeping sickness. What’s more, at high enough concentrations, the drug can kill mammalian cells, too, which means it will need to be carefully tested for toxicity.
“There are all these hurdles before it can be used as a drug,” Steverding cautions. “It’s not that they have a magic bullet.” If the specificity, and thus the safety, of the drug can be improved, it might be a good candidate for further development, he says.






