Quick-Change Surface: Material repels water on command
In an advance that could open new routes to sensors, drug delivery, and many other technologies, researchers have modified a gold surface so that it switches from a water-attracting mode to a water-repelling one on command. With that capability, a flip of a switch could cause drug molecules, proteins, or cells to collect on the surface or to suddenly be released from it.
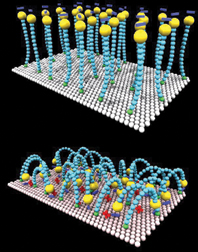
The turncoat layer, atop a gold-coated wafer, is made of neatly aligned, chainlike molecules that stand up like bristles on a brush. The molecules’ tips exposed to the world are negatively charged and attract water, but the molecules’ midsections shun water. When scientists apply an electric field to the wafer, the gold attracts the molecules’ negative tips and each bristle bends over, exposing its hydrophobic belly.
Since no chemical bond breaking or bond forming occurs, the process is easily reversible, says Joerg Lahann of the Massachusetts Institute of Technology (MIT). He and his colleagues from MIT and the University of California, Santa Barbara and Berkeley describe their surface in the Jan. 17 Science.
Making the surface took some computation and a neat chemical trick. When the bristle molecules assemble themselves onto a gold surface, they usually pack together so tightly that they can’t bend over. To overcome this problem, Lahann and his coworkers ran simulations that showed that each molecular bristle needs at least 0.65 square nanometer to bow. So, the team found a chemical group that takes up about
0.67 square nanometer of space and linked it to the negatively charged tip of the molecular bristles. Once these mushroom-shaped molecules assembled on the gold, they each occupied about 0.67 square nanometer. When the scientists chemically lopped off the molecules’ space-filling heads, each bristle had enough room to bend over.
“This is quite creative work,” comments chemical engineer Manoj K. Chaudhury of Lehigh University in Bethlehem, Pa. For use in many technologies, the technique needs to produce a surface that is even more repellant to water than those in the tests so far, he notes. Then the method would prove useful, for example, in microfluidic devices in which fluid must be reliably directed through exquisitely fine pathways.
****************
If you have a comment on this article that you would like considered for publication in Science News, please send it to editors@sciencenews.org.







