Scientists get a handle on crystal shape
- More than 2 years ago
In a discovery that could have implications ranging from the design of new materials to an understanding of the origin of life, researchers have discovered how the microscopic orientation of amino acid molecules can force a crystal to take on either a left- or right-handed form.
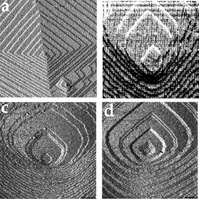
Researchers have known for years that organic molecules can glom onto a growing crystal’s facets and influence the crystal’s shape. Until now, however, no one knew quite how. The new results, reported in the June 14 Nature, show how amino acids work at the molecular level to accomplish this feat.
Like gloves, many individual mineral crystals or organic molecules exist in two mirror-image forms–a phenomenon known as chirality. A month ago in the Proceedings of the National Academy of Sciences (PNAS), other researchers reported that microscopic steps on certain calcite-crystal faces have an affinity for either right- or left-handed amino acids (SN: 5/5/01, p. 276: Rocks May Have Given a Hand to Life).
The new work also shows a close relationship between amino acids and calcite’s tiny steps. Christine A. Orme of the Lawrence Livermore (Calif.) National Laboratory and her colleagues used atomic-force microscopy to study calcite-crystal surfaces. When the researchers added amino acids to a solution in which the crystals were growing, the microscopy revealed that some of the crystals’ tiny, straight steps became curved.
Even more intriguing, the researchers found that the addition of the left-handed version of aspartic acid, an amino acid, created a step pattern in calcite crystals opposite to that seen with the addition of the right-handed version.
The researchers followed their microscopy with computer modeling that revealed a possible mechanism for aspartic acid’s effects on calcite–the presence of amino acids alters a step’s energy. That energy dictates how much a step curves, says team member James J. De Yoreo of the Livermore lab. Since left- and right-handed amino acids bind preferentially to opposite steps, the steps curve more in one direction based on which handed form is present, he says.
Living organisms might use such a mechanism to create chiral crystals, such as those that aggregate into shells, the researchers propose. The finding even has implications beyond earthly life, says De Yoreo. Astrobiologists now might look at crystal formations in meteorites and tell whether their forms are predominantly left- or right-handed, he suggests. Since amino acids in living organisms on Earth are almost exclusively of the left-handed variety, such a finding in meteorites could suggest that amino acids had influenced the crystals’ shapes, hinting at extraterrestrial life.
Another possibility: Materials scientists might eventually harness the shape-directing power of organic molecules for designing new materials or for guiding the growth of new structures for nanoscale devices.
Robert Hazen of the Carnegie Institution of Washington (D.C.), a coauthor of the earlier PNAS report, agrees. “Life uses this [mechanism] in developing its own nanotechnology,” he comments. “So why not use this in nanoengineering?”







