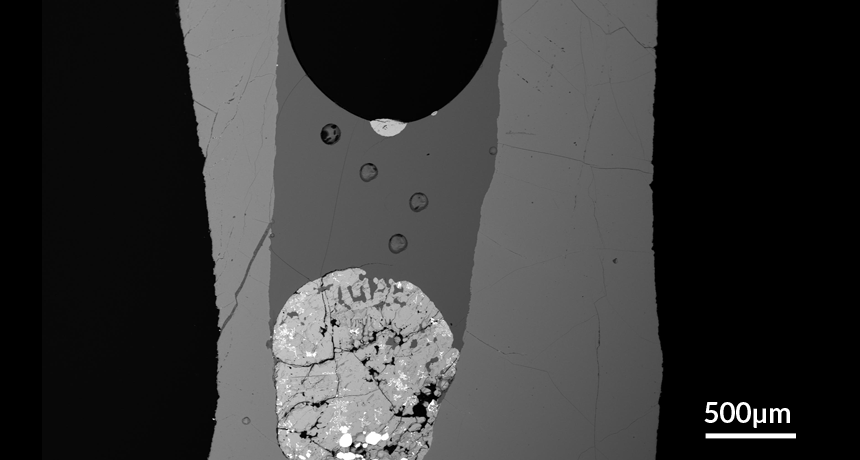
BUBBLING UP Tiny droplets (white semicircles) from a dense mixture of sulfur and metal (glob at bottom) can float upward in magma reservoirs on the undersides of steam bubbles (black circle), new research suggests.
J.E. Mungall et al.
Dense mixes of sulfur and metals such as gold and copper can catch a lift to the top of magma reservoirs on the undersides of rising steam bubbles, new research suggests.
After heating capsules of materials commonly found in magma, researchers discovered heavy droplets of sulfur and metal stuck to the bottom of floating water vapor bubbles. The mechanism may explain the large amounts of sulfur and metals expelled during volcanic eruptions and help miners pinpoint undiscovered ore deposits, the researchers propose February 23 in Nature Geoscience.
“These little floaters are pretty cool,” says lead author and geoscientist James Mungall of the University of Toronto. “They’re like little hot air balloons carrying a gondola underneath.”
Metals in magma readily combine with any nearby sulfur “to a shocking degree,” Mungall says. Sulfur-rich melts will typically have 10,000 times the gold concentration of the surrounding magma, for instance. This dense combination of sulfur and metal, known as a sulfide mineral, will sink and sit indefinitely at the bottom of the magma reservoir.
But volcanologists have seen volcanic blasts, such as the 1991 Mount Pinatubo eruption in the Philippines, spew more sulfur and metal than could be carried to the surface in the erupted magma. This imbalance suggested that some unknown mechanism can raise sulfur and metal from deep inside the Earth.
Mungall and colleagues weren’t looking to solve this mystery when they began an experiment mimicking how sulfide minerals behave in magma chambers. The team first drilled millimeter-wide holes in large crystals of heat-resistant chromite and packed the cavities with magma ingredients such as silicate and iron sulfide. The vessels then went into a furnace preheated to 1,200° Celsius. After 48 hours, the team quickly cooled the creations, freezing the molten interiors in place.
When the researchers sliced open and inspected their mock magma, they discovered dense droplets of sulfide minerals hanging from the bottom of floating steam bubbles. “It was surprising the first time we noticed it,” Mungall says, “but then it started to make sense.”
A bubble will morph its shape to minimize the amount of surface tension energy required to remain a bubble, with a sphere requiring the least amount of energy to hold together. When two bubbles meet, their surfaces will merge to form a shared wall that reduces the total surface area that surface tension must maintain. Mungall believes this energy-reducing behavior bonds the sulfide mineral droplets and steam bubbles together as they float potentially thousands of meters up the magma reservoir. Over time, the steam bubbles, which range in size from microscopic to several meters across, will absorb their hitchhiking payloads and either erupt to the surface or settle into fissures, forming near-surface metal deposits.
The explanation is “excitingly tidy,” says geologist Adam Simon of the University of Michigan in Ann Arbor. “We’ve known for a long time that sulfides and water vapor bubbles exist in these magma chambers, so coupling the two provides a beautiful and simplistic way to transfer the sulfur and metals to the overlying environment.”If commonplace, the newly discovered mechanism could help mining companies locate undiscovered deposits of copper, gold and other valuable metals, Simon says.