These tiny aquatic animals secrete a compound that may help fight snail fever
A molecule made by rotifers prevents parasitic worm larvae from causing infections in mice
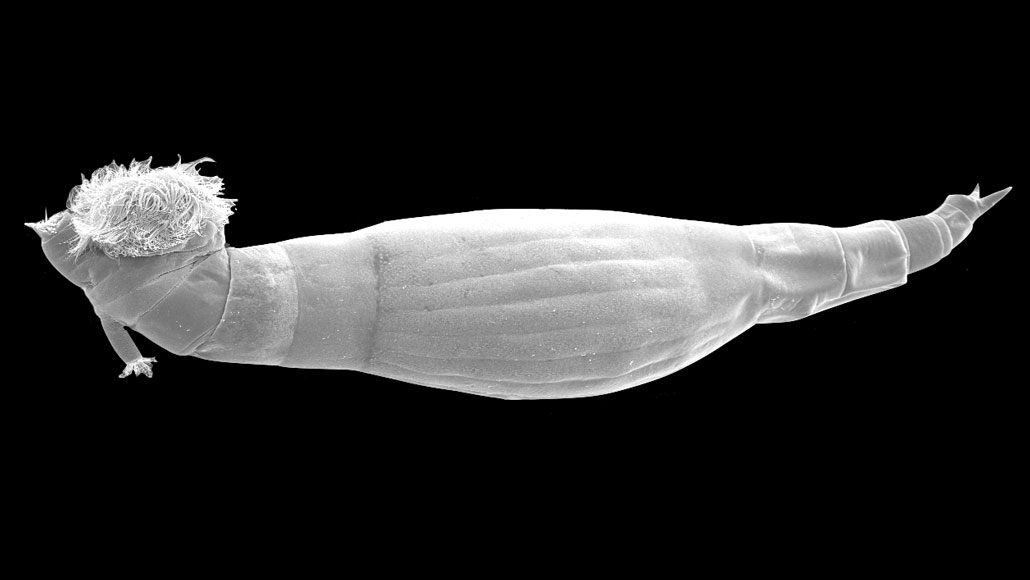
The rotifer Rotaria rotatoria (shown in a microscope image) produces a newly identified molecule that paralyzes the larvae of worms that cause schistosomiasis, preventing them from spurring new infections.
Newmark Lab