Alzheimer’s protein can travel from blood to build up in the brain
Movement of amyloid-beta could suggest new ways to treat the disease
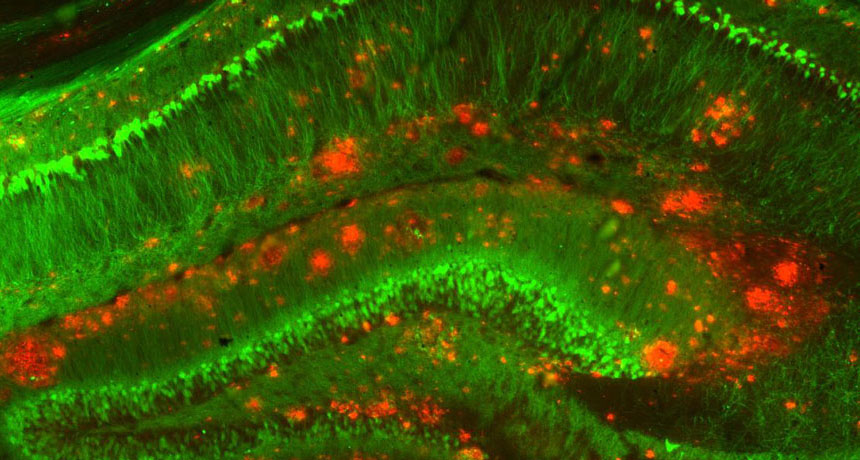
BRAIN BUILDUP One of the changes to brains with Alzheimer’s disease is the buildup of clumps of a protein called amyloid-beta (red) among nerve cells (green, mouse brain shown).
Strittmatter Laboratory/Yale University
An Alzheimer’s-related protein can move from the blood to the brain and accumulate there, experiments on mice show for the first time.
The results, published online October 31 in Molecular Psychiatry, suggest that the protein amyloid-beta outside the brain may contribute to the Alzheimer’s disease inside it, says Mathias Jucker, a neurobiologist at the University of Tübingen in Germany. This more expansive view of the disease may lead scientists to develop treatments that target parts of the body that are easier than the brain to access.
The experiments don’t suggest that people could contract Alzheimer’s from another person’s blood. “The bottom line is that this study is thought-provoking but shouldn’t cause alarm,” says neurologist John Collinge of University College London. “There really isn’t any evidence that you can transmit Alzheimer’s disease by blood transfusion.”
But researchers wondered whether, over time, A-beta might build up in the brain by moving there from the blood, where it’s normally found in small quantities. Earlier animal studies have shown that A-beta can move into the brain if it’s injected into the bloodstream, but scientists didn’t know whether A-beta from the blood can be plentiful enough to form plaques in the brain.
To test this, researchers used a form of extreme blood-sharing in the experiment. Six pairs of mice — with one mouse engineered to produce gobs of human A-beta and one normal — were surgically joined for a year, causing blood mingling that’s far more extensive than that of a blood transfusion. After a year, the brains of the mice carrying the mutations were full of A-beta plaques, as expected. But these plaques were also inside the brains of the normal mice in the joined pairs.
Story continues after image
Plaque share
The brains of mice mutated to produce large amounts of amyloid-beta developed plaques (arrowheads, right). Mice that shared blood with these mutant mice also had plaques (middle), while control mice did not (left).
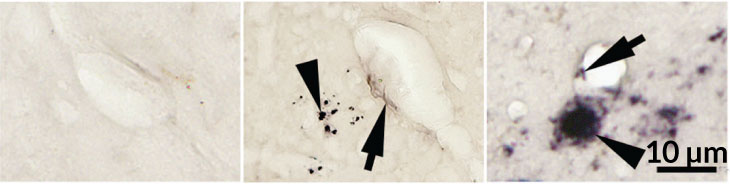
In those normal mice, A-beta levels weren’t as high as they were in the mutated mice, but the fact that they existed was notable, says study coauthor Weihong Song of the University of British Columbia in Vancouver. Unjoined control mice without the mutation showed no A-beta accumulation.
The brains of the joined mice also showed other signs of deterioration. The researchers observed inflammation, tiny areas of bleeding and a dangerous type of the protein tau in the brains of normal mice that had been exposed to blood full of A-beta. In people, Alzheimer’s is often marked by both A-beta plaques and tangles of tau.
The results don’t mean that Alzheimer’s is predominantly caused by factors in the blood. “We still think of Alzheimer’s as a brain disorder,” Song says. But factors in the blood, in some cases, might have the power to nudge the disease along, the results suggest.
A-beta is made by cells in the brain, but also by blood platelets, skin cells, muscles and other parts of the body. Normally, “there is a balance between A-beta inside and outside the brain,” Song says. But when this balance is thrown off, such as when the body is chock-full of the protein, or when the blood-brain barrier — the blockade that keeps potential dangers out of the brain — deteriorates with age, the brain may get an extra dose, Song proposes. By tweaking this balance, it’s possible that drugs or therapies that reduce A-beta in the body might help slow or prevent Alzheimer’s disease.
Evidence has been accumulating that A-beta can behave as a prion, a misfolded protein that can incite normally folded proteins to go rogue (SN: 10/17/15, p. 12). Song says that the experiments don’t address whether A-beta from the blood can behave as a prion and prompt already existing A-beta in the brain to form plaques. The normal mice’s brain plaques seemed to be built from human A-beta protein, and the only source of that was the blood of the mutated partner mouse.