Ancient Extract: T. rex fossil yields recognizable protein
New analyses of a Tyrannosaurus rex leg bone reveal that the fossil, which is 68 million years old, preserves substantial remnants of proteins. The chemical makeup of the protein fragments adds to earlier evidence that modern birds are closely related to dinosaurs.
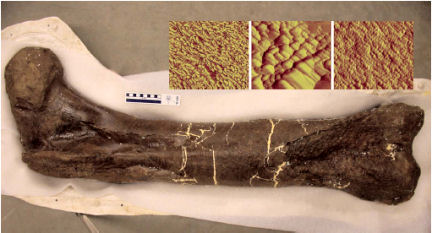
Most animal fossils include only hard body parts, such as teeth and bones, and until recently, few paleontologists dared hope that soft tissues could survive in long-dead remains. Recently, however, a team of researchers—including Mary H. Schweitzer, a paleontologist at North Carolina State University in Raleigh—extracted soft, pliable tissue from the fossilized leg bone of a T. rex (SN: 3/26/05, p. 195: Old Softy: Tyrannosaurus fossil yields flexible tissue).
Now, further analyses of samples from that leg bone have identified small fragments of the long, fiber-forming protein collagen, the major nonmineral component of living bone.
Several lines of evidence point to collagen, says Schweitzer. First, microscopic images of bone samples showed structural features spaced about 70 nanometers apart, matching the scale of tiny structures that collagen forms in living creatures. Second, the chemical analysis of bone samples identified many of the amino acids found in collagen. The most common of those substances, glycine and alanine, appeared in a 2.6-to-1 ratio, similar to the 2.5-to-1 ratio of the substances in the collagen of chickens.
Finally, antibodies that react with a certain type of chicken collagen also reacted to a powdered and purified sample of the T. rex bone, says Schweitzer. She and her colleagues report their findings in the April 13 Science.
The chemical composition of collagen varies from species to species, says coauthor John M. Asara, an analytical chemist at Harvard Medical School in Boston. However, even in disparate creatures, the specific sequences of the amino acids in some types of collagen can be remarkably similar. For example, 97 percent of the amino acid sequence in human collagen matches that of the cow.
Asara, Schweitzer, and other team members used mass spectrometry to identify the sequence of protein fragments in collagen from the T. rex fossil. That was a difficult challenge, since each 30-to-40-milligram tissue sample had just a few nanograms of collagen, says Asara. Nevertheless, the researchers identified seven sequences of between 10 and 20 amino acids that matched those found in the collagen of modern-day creatures.
The amino acid sequences of the T. rex collagen more closely resembled sequences found in chickens than those found in the other current animals that the team examined. In contrast, sequences of amino acids from collagen extracted from a mastodon skull that’s between 160,000 and 600,000 years old are more similar to those of mammals than to those of other current animals, says Asara. The researchers report these results in the same issue of Science.
Analyses of fossils from many different geologic periods will reveal whether protein preservation is exceptional or commonplace, says paleontologist Derek E.G. Briggs of Yale University. If such preservation is far more frequent than paleontologists have expected, discerning the amino acid sequences in ancient proteins “has enormous potential” for revealing evolutionary relationships among ancient creatures, he adds.