People with rare blood clots after a COVID-19 jab share an uncommon immune response
Some who get AstraZeneca’s or Johnson & Johnson’s shots make antibodies that spark clots
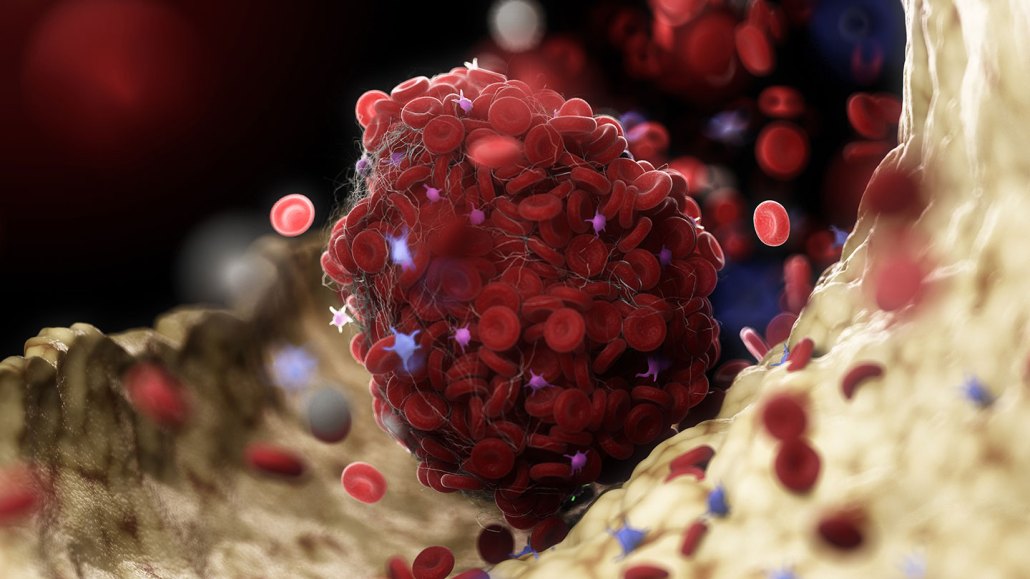
Antibodies that bind to a protein called platelet factor 4 may be behind rare, but dangerous, blood clots (one illustrated) that develop in some people vaccinated with AstraZeneca’s or Johnson & Johnson’s COVID-19 vaccines.
SEBASTIAN KAULITZKI/SCIENCE PHOTO LIBRARY/Getty Images







