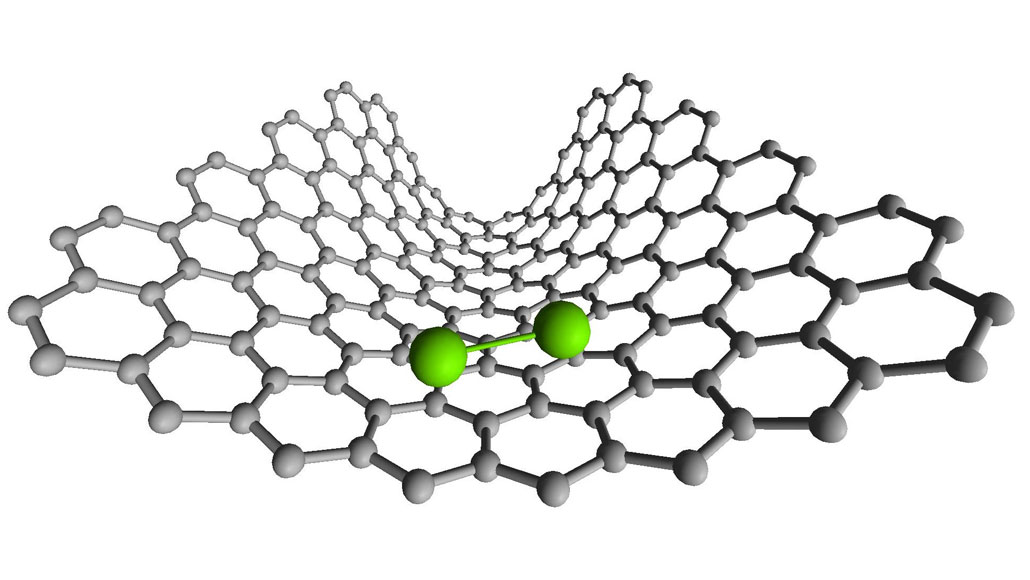
Scientists spied on two atoms forming chemical bonds and breaking them by studying molecules made of two rhenium atoms (illustrated in green) inside a carbon nanotube (gray).
University of Nottingham

Scientists spied on two atoms forming chemical bonds and breaking them by studying molecules made of two rhenium atoms (illustrated in green) inside a carbon nanotube (gray).
University of Nottingham