Evaporating mixtures of two liquids create hypnotic designs
Signature patterns are the result of differing surface tension
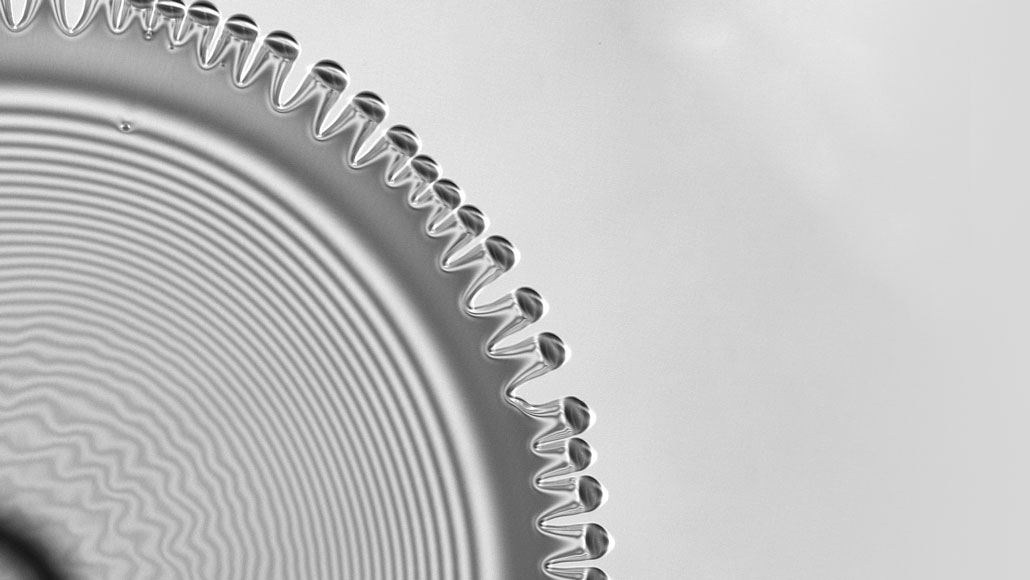
Droplets containing liquids with different surface tensions, like isopropanol and ethylene glycol (shown), bloom into intricate patterns as they evaporate.
A.P. Mouat et al/Physical Review Letters 2020