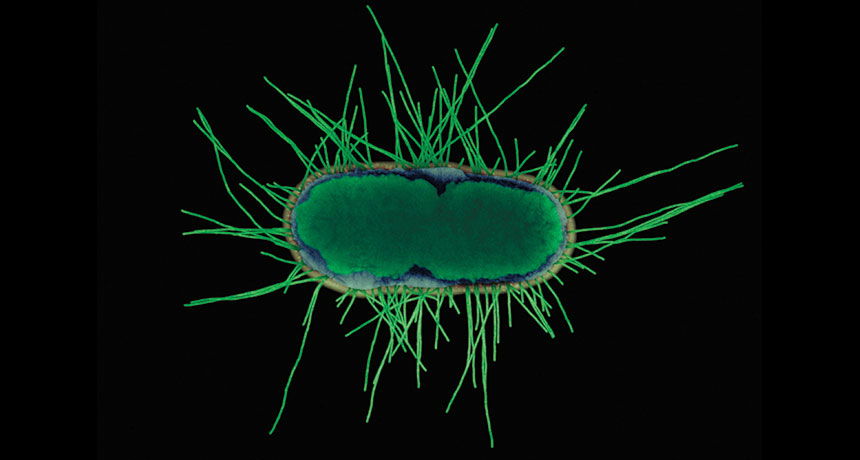
LOSS OF APPETITE Experiments show that helpful gut bacteria like this E. coli K12 produce proteins that could influence the appetite of mice and rats.
Kwangshin Kim/Science Source

LOSS OF APPETITE Experiments show that helpful gut bacteria like this E. coli K12 produce proteins that could influence the appetite of mice and rats.
Kwangshin Kim/Science Source