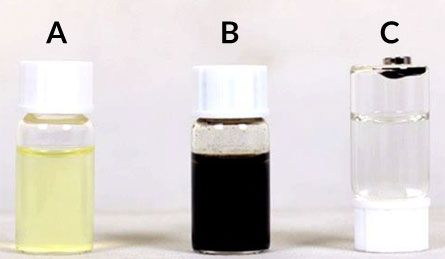
Iron nanoparticles snatch uranium
To retrieve the radioactive loot, scientists just need a magnet
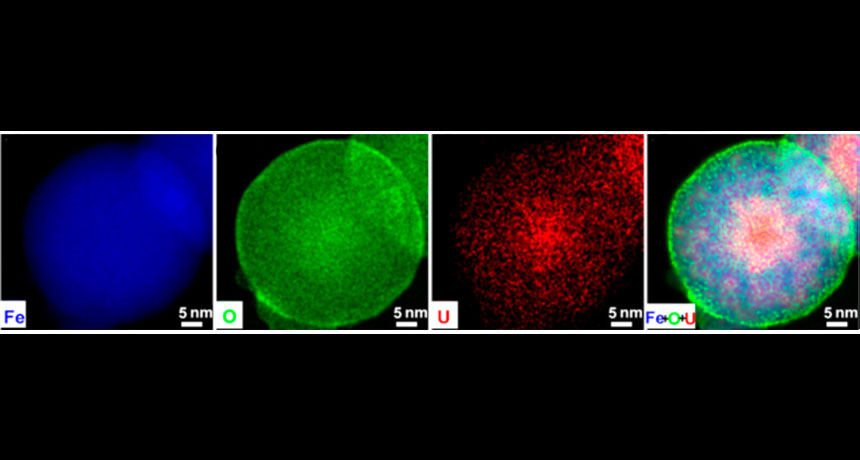
TAKING THE BAIT In an iron (FE) nanoparticle (blue) with an iron hydroxide shell containing oxygen (O, green), uranium (U, red) first takes the bait created by the shell and then is trapped inside the solid iron core.
Adapted with permission from L. Ling and W. Zhang/Journal of the American Chemical Society 2015