A winter expedition into frigid Antarctic waters is no pleasure cruise. Screaming winds, bone-chilling temperatures, high seas, driving snow, and crunching ice create conditions that try the hardiest of souls.





It’s an unlikely place to find a mathematician.
Last year’s 8-week voyage of the ice-breaking research vessel Aurora Australis from Tasmania wasn’t Kenneth M. Golden’s first venture into the Antarctic ice pack. An applied mathematician at the University of Utah in Salt Lake City, he had also studied sea ice on three previous Antarctic outings.
Foul weather never kept Golden from relishing an Antarctic voyage. “I loved watching the waves,” he says. When the sun squeaked through storm clouds during the few hours of daylight, he could also enjoy spectacular views of giant icebergs, crumpled ice sheets, and penguins waddling across snow or skimming the water.
About four dozen researchers were aboard the icebreaker on its 1999 voyage. Their destination was a sizable region, called a polynya, of open water and thin ice that had appeared in the ice-bound waters near the toe of the Mertz Glacier off the Antarctic coast.
The sea-ice pack insulates the ocean from the atmosphere. A hole in this lid permits a significant amount of heat to escape from the water to the much chillier air. At the same time, surface waters cool, become denser, and sink, contributing to ocean circulation (SN: 7/15/00, p. 42: Available to subscribers at The Motion in the Ocean). New ice begins to form, filling in the hole.
As the boundary layer between ocean and atmosphere in polar regions, sea ice has a significant impact on global climate, Golden notes. Polynyas play a crucial role in energy exchange and ice formation, so the occurrence of one inevitably draws the attention of researchers.
On a broader front, sea ice “is most important in the context of climate variability and change, both as an indicator and an agent of climate change,” says geophysicist Hajo Eicken of the University of Alaska in Fairbanks.
The need to understand better the physical properties of sea ice, track its movements, and remotely monitor its thickness, temperature, and roughness has prompted a number of recent studies of its structure.
In May at a Society for Industrial and Applied Mathematics meeting in Philadelphia on mathematical aspects of materials science, Golden described how new mathematical models have provided insights into the way brine seeps through ice, carrying heat and nutrients.
Other models have also helped researchers refine methods for reconstructing the physical characteristics of polar ice—its age, thickness, roughness, brine content, and porosity—from satellite or airplane measurements of microwaves bounced off the surface.
Mathematical and computer models—both simple and complex—help researchers design field experiments and provide insights that can guide the development of accurate, large-scale climate models, Eicken says.
Remarkable scene
It was during Golden’s 1994 trip to the Antarctic—his second expedition—that he witnessed a remarkable scene that was to motivate much of his subsequent mathematical research on sea-ice structure.
Buffeted by winds reaching 60 miles per hour during a fierce snowstorm, he was out on the ice in the middle of the night. “It was snowing like crazy, but it was warm,” Golden recalls.
What caught his eye was a massive upwelling of liquid, percolating up through the ice to flood the surface beneath the newly fallen snow, forming a slushy boil. Though he had heard about this phenomenon and had even proved theorems about percolation models, he had never before seen it or realized how striking the phenomenon is.
“That was very important for guiding my theoretical research and suggesting new questions to investigate,” Golden remarks.
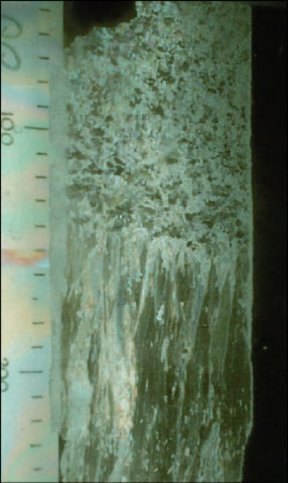
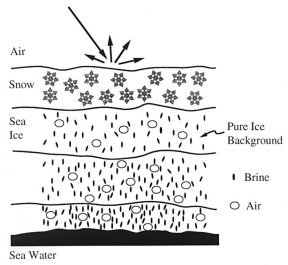
To understand percolation, he had to dig into sea-ice formation. As seawater freezes, it can’t incorporate salt into its crystal structure. The salt that it rejects concentrates in brine pockets between crystals. So, sea ice is a porous material made up of pure ice laced with brine-filled cavities and air bubbles.
Unlike other porous materials, such as sandstone or bone, sea ice’s microstructure and bulk properties can change dramatically over a small temperature range. Sea ice becomes permeable and brine can travel through the solid when temperatures rise above about -5ºC, if the brine-volume fraction is 5 percent and the salt content is 5 parts per thousand. At lower temperatures, sea ice is effectively impermeable, locking in place any liquid that happens to be present.
In polar regions, a snowstorm can elevate the ice temperature and push down on the surface. Brine cavities grow larger and connect, so the ice becomes permeable. Sea water percolates up through the ice to flood the surface and form the boils observed by Golden.
Interest in assessing brine movement through ice has escalated in recent years as researchers have come to recognize the vital role of percolation in polar ecosystems. It supplies nutrients to algal communities and bacteria living in the ice, transports contaminants, and heats the atmosphere.
To develop a mathematical model of brine transport, Golden turned to percolation theory. In the simplest form of a percolation model, one considers an array of points arranged in a square lattice or a cubic network. The idea is to introduce links between randomly chosen pairs of neighboring points until it’s possible to travel from one side of the grid to the other along an unbroken chain.
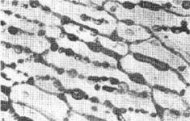
The microstructure of a compressed powder made up of large polymer particles and small metal particles (left) resembles that of sea ice (right). (Golden et al./Science) | |
In two dimensions, such a pathway appears when roughly half of all possible links are in place; in three dimensions, about one-quarter of the links must be present. If ice is regarded as a random mix of brine pockets and ice particles of equal size, the simple percolation model says sea ice should be at least 25 percent brine before salty water can percolate through it. The actual figure for sea ice is 5 percent, however.
Golden solved this puzzle by turning to a more sophisticated percolation model, first used in the 1960s to study the electrical behavior of metal particles mixed with a nonconducting polymer. Engineers had found that by compressing powders made up of large polymer particles and much smaller metal particles, they could obtain a flexible, composite material that permitted electricity to flow despite a relatively low metal content.
The microstructure of such a compressed powder is remarkably similar to the cellular microstructure of the columnar form of sea ice, Golden remarks. The most important parameter for the powder model is the ratio of the radius of the large particles to that of the smaller ones.
Applying the compressed-powder-conductivity model to typical sizes of ice crystals and brine cavities in columnar sea ice yielded a critical brine volume of about 5 percent.
The model, however, serves only as a rough approximation of both brine percolation and ice structure. In reality, the situation is much more complicated. For instance, once percolation begins, brine pockets quickly connect with each other to create large-diameter brine channels along which most of the fluid transport takes place.
During the 1999 voyage to Antarctica, Golden, Victoria I. Lytle of the Antarctic Cooperative Research Centre in Hobart, Australia, and their coworkers ventured out onto the ice to examine brine channels in the field. They used chain saws to cut out slabs of ice 15 to 26 inches thick. Beet juice scrounged from the ship’s galley revealed the intricate brine pathways threading through the ice.
“As beet juice seeps into a slab, you see layers of different types of ice crystals, and you get a feel for how brine moves through the ice,” Golden says. The researchers are now trying to determine precisely how these brine channels form.
Laboratory measurements and field studies by Eicken and his coworkers have revealed other aspects of sea-ice behavior. Even at low temperatures, some liquid can slowly seep through the solid, Eicken notes. “It will be interesting to have a closer look at some of the processes that help maintain connected pore space even at low temperatures.”
Another important issue is how the microphysics of sea-ice structure determines the extent and quality of microbial activity in the ice. Researchers need such information to assess the role of sea-ice organisms in taking up carbon in the Arctic and Antarctic.
Structural details can be important. “Given the microscopic size of the organisms, even a few connected pores, which do not affect the macrophysics of the system measurably, can decide the fate of a group of organisms,” Eicken remarks.
Don Perovich of the U.S. Army Cold Regions Research and Development Center in Hanover, N.H., says, “Here, we have a frozen environment that is home for a rich and diverse food web. Understanding life in this extreme environment may provide insights in the search for extraterrestrial life.”
Vivid demonstration
In late August during the 1999 expedition, Golden and five colleagues got a vivid demonstration of sea-ice dynamics on a larger scale.
The researchers had just descended to the ice to collect samples, when a small crack appeared, then quickly widened. Four people crowded back into the small cage that had brought them down, and the ship’s crane hoisted them back on board, leaving Golden and researcher Robert A. Massom of the Antarctic Cooperative Research Centre on the ice to await the cage’s return.
Meanwhile, the crack closed, and a slab of ice started riding up over the slab on which Golden and Massom were standing, pushing it down so that sea water began flooding its surface. After what seemed like an interminable wait, the crane finally dropped the cage for the second pickup. Two minutes later, the ice sheet had broken up completely.
“It’s plate tectonics on a very fast timescale,” Golden observes.
Experiments and measurements on the ice surface provide important data for calibrating mathematical models and interpreting remote-sensing signals. The extent of the connections among brine cavities, for instance, affects how sea ice interacts with electromagnetic radiation, and surface flooding and subsequent freezing influence how a surface scatters microwaves.
In remote sensing, it’s often difficult to distinguish between slush and open water. A better understanding of slush formation would allow researchers to refine remote-sensing methods to distinguish different types of ice and open water more reliably.
Hence, knowledge of how brine flows is important for accurate satellite measurements, Golden says. Such data, in turn, would provide information on the extent and thickness of polar ice, a parameter useful in tracking global climate change.
The mathematics behind techniques for interpreting reflected microwave signals is interesting, Golden says. “We now have a lot of new results, which can also be applied to other types of scattering.”
Penguins enliven an Antarctic ice field. |
Traveling to the Antarctic ice pack to collect data also has dangers unrelated to the ice.
On its 1998 voyage, after sailing for a week, the Aurora Australis finally reached the ice edge, 100 miles off the Antarctic coast. Late that night, a serious engine-room fire triggered alarms throughout the ship, bringing everyone on deck.
Awoken soon after he had gone to sleep, a groggy Golden showed up wearing just a light jacket and jeans. Luckily, although lifeboats were lowered, the ship didn’t have to be abandoned. A few hours in a lifeboat at temperatures nearing -65ºC would have made the folly of Golden’s choice of apparel painfully clear.
“I should have known better,” he admits.
Eventually, crew members managed to control the fire, though it ended up gutting the main engine room. Engineers jury-rigged a control system for a backup engine, and the ship slowly limped 3,000 kilometers back to port.
Unfazed by his chilling experience and still in pursuit of all things ice, Golden was back on board in 1999 for a return trip to Antarctic waters.