Chemists have taken a tip from Tony Soprano — where cajoling, fear and intimidation fail, brute force may succeed. To break the strong bonds of an exceptionally stable ring-shaped compound known for resisting all powers of chemical persuasion, researchers attached chains to it and physically tore the thing apart.
The extremely sturdy compound, a triazole ring found in many drugs and fungus-fighting chemicals, was yanked asunder using molecular chains and the power of suction that’s created when bubbles implode. This new approach, published in the Sept. 16 Science, suggests a powerful new means for bossing molecules around.
“It’s a way to almost literally put your hands on molecules and twist them or turn them in whatever way you want,” says chemist Christopher Bielawski of the University of Texas at Austin, coauthor of the new study.
The new approach might prove useful for making stress-detecting sensors that can be embedded in plastics and other materials, or bodyguard molecules that protect parts of compounds in particular chemical environments and then leave when they are directed to do so. The research also suggests that some existing drugs thought to be stable in the body may actually be vulnerable to enzymes that tug and push molecules into place during reactions, says Bielawski. Such breakage could produce potentially toxic breakdown products.
“This work is going to have a big impact,” says chemist Virgil Percec of the University of Pennsylvania. “It opens the door to unexpected new opportunities.”
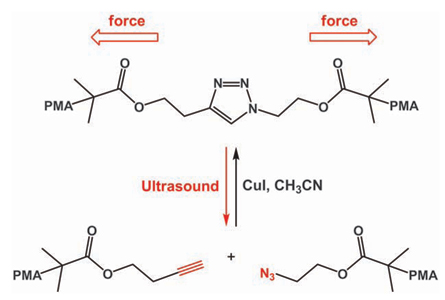
Bielawski and two of his students, Johnathan Brantley and Kelly Wiggins, were trying to reverse a prominent “click chemistry” reaction that combines two versatile chemical building blocks, an azide and an alkyne, to make the triazole ring. The beauty of click chemistry reactions is they bring together molecules cleanly and efficiently, like a seat belt buckling.
“Click chemistry is a great reaction for stapling things together, but you could never take the staple out,” says materials chemist Jeff Moore of the University of Illinois at Urbana-Champaign.
Chemists usually use heat, light or molecular helpers known as catalysts to undo compounds, but these methods open doors to all sorts of reactions that can result in end products you don’t want. And those tricks don’t work on the triazole ring anyway, because it’s too tough. Cooking a triazole ring for several hours at 300° Celsius does nothing.
“This ring is like a rock,” says Moore. “It is so robust, nobody’s been able to figure out how to get it to come apart.”
The researchers accomplished this feat by attaching polymer chains to opposite sides of the triazole rings. Then the team inserted an ultrasound probe in the solution, generating bubbles that at a certain size start imploding. These implosions create tiny vacuums. The liquid solution rushes into these black hole–like voids, yanking on some of the polymer chainsand tearing open the rings.
“It’s a very cool result,” says Moore. “When something like this comes along, you think ‘Why didn’t I think of that?’”
And the chains are easy to remove, so the remaining azide and alkyne components can be used for anything: even making another triazole ring.






