A block of ice melts at 0Ë Celsius. But chip a few molecules off that ice block and they will begin to melt at a much lower temperature — almost 180 degrees cooler.
Nanoparticles of ice were thought to melt at much lower temperatures than ice in bulk, but not this low, say researchers from Germany, France and China in a report to appear in Physical Review Letters.
“This is a pretty big deal for understanding water,” comments physicist H. Eugene Stanley of Boston University.
“Water is a really difficult material” to model, says Bernd von Issendorff, a coauthor of the new paper and a physicist at the University of Freiburg in Germany. “And because we don’t really understand bulk water, we don’t understand nanoparticles.”
Water nanoparticles, tiny clusters of H2O molecules, are known to be important in the Earth’s atmosphere and in interstellar space. Understanding how nanoparticles of water behave — including their melting points — could shed light on systems characterized by nano-sized volumes of water, such as the trace amounts found inside cells, Stanley says. “Some biologists think we won’t fully understand biology until we understand water,” he says.
Whereas most drops of liquid behave roughly like spheres, a drop of liquid water behaves “like a bag of jacks” with charged tips that attract and repel each other, Stanley says.
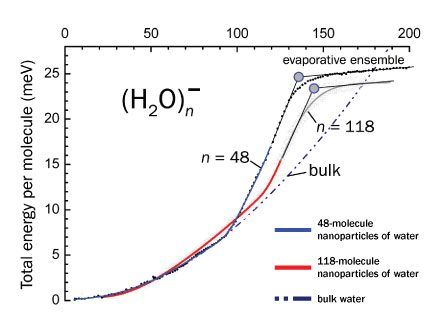
To tease out the melting point, von Issendorff and colleagues plotted the internal energy of water nanoparticles against temperature. The researchers used a method called photofragmentation to knock some of the molecules off the tiny clusters with a laser, which enabled the scientists to determine the clusters’ internal energy.
The researchers used clusters of ice consisting of either 48 or 118 water molecules. The relationship between the internal energy and temperature for these ice nanoparticles mirrored that of bulk ice at cooler temperatures. But beginning at roughly 93 kelvins (-180ËC) for the 48-molecule clusters and at 118 K (-155ËC) for the 118-molecule clusters, the nanoparticles’ energy relative to temperature rose steeply in comparison to that of the bulk ice.
The sudden change in the relationship between internal energy and temperature indicates a phase transition, or melting point, von Issendorff says.
But the transition temperature the team found is not a “textbook” melting point, von Issendorff says. The transition from solid to liquid takes place over a range of 20 to 40 degrees, implying that the transition is more gradual for a nanoparticle than for bulk ice, he says. Instead of changing almost immediately from a frozen crystal lattice to the randomly moving particles of a liquid, the nanoparticles seem to get gradually softer as they get heated. “Something is happening that takes more energy, but we don’t see a sharp step that you’d see in a typical phase transition,” von Issendorff says.
The fact that the smaller 48-molecule particles have a lower melting temperature than the larger 118-molecule particles indicates that there is a direct relationship between cluster size and melting point, which might lead the way to a phase transition model that applies to all forms of water, including nanoparticles, Stanley says.






