Nurturing Our Microbes
Stewardship of the life teeming within us can pay health dividends
Each of us is a metropolis. Bustling about in everyone’s body are tens of trillions of microbes. Some are descended from starter populations provided by mom during birth. Additional bacteria, yeasts, and other life forms hitchhike in with foods. By age 3, everyone’s gut hosts a fairly stable, yet diverse, ecosystem.

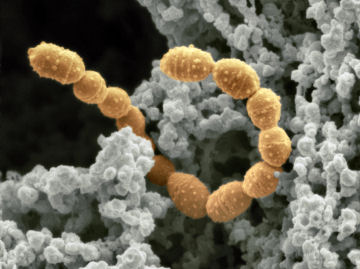
Most of the tiny stowaways hide out in the gastrointestinal tract—the gut-stealing a share of everything we eat or drink. But that’s only fair, because most of these bugs give as good as they take, explains microbiologist Jeffrey I. Gordon. They not only help us digest food, he says, but they also harvest nutrients, manufacture certain vitamins, kill germs, neutralize bacterial toxins, and modulate the immune system. Sickness, antibiotic therapy, or stress, however, can disrupt the ecological balance among gut dwellers—known as flora—diminishing their benefits.
Because these benefits are vital to health—and to averting disease—drug manufacturers are eyeing gut microbes as potential therapeutic targets. In the future, “pharmaceutical companies might be drugging your bugs, not drugging you,” suggests Jeremy Nicholson of Imperial College, London.
In the meantime, over-the-counter therapies exist to bug, not drug, the bugs. Known as probiotics, these yogurts and other foods or dietary supplements introduce or replenish beneficial gut species in the digestive system (SN: 2/2/02, p. 72).
Probiotic microbes’ role in fighting generic diarrheal disease is old hat, but in the past decade, other influences on human immunity and metabolism have emerged. Certain microbial supplements show the potential to reduce the severity of colds and other infections, temper body weight, and even help the elderly fight osteoporosis.
The rub: Research is showing that a probiotic’s benefits can be very specific. In fact, it might be more appropriate to view these microbes as a cornucopia of diet-based, over-the-counter micro-pharmacists—each able to dispense only a few therapies or services.
But for all the promise that probiotics offer, they’re no panacea, many researchers caution, and may even exhibit disturbing effects (see “Not without Risks,” below). Within a given species, some strains may confer health benefits, others may not.
Yet when the right bug is ingested for a particular condition, even a small dose can trigger dramatic health benefits.
Dining partners
“The total number of microbes associated with our adult bodies exceeds the total number of our human cells by a factor of 10,” says Gordon, of Washington University in St. Louis. So effectively, “we’re sort of a superorganism—one that’s 90 percent microbial.”
Other animals have evolved a similar symbiosis with—or even dependence on—gut microbes, the scientist notes. Rodents born by cesarean section (so they get none of their moms’ intestinal flora) and raised under germfree conditions end up smaller than normal, his group found—despite eating “about 30 percent more food than their microbe-laden counterparts.”
Germfree animals not only appear less efficient at harvesting calories, he explains, but also “are prone to certain vitamin deficiencies” because gut microbes synthesize certain nutrients, such as vitamins B12 and K.
Gut flora also help the body mine minerals from the diet. “We have measured this for calcium,” says Jürgen Schrezenmeir of Germany’s Federal Research Center for Nutrition and Food, in Kiel.
His team showed that supplementing rats’ diets with a probiotic strain of bacteria, Lactobacillus acidophilus, kept the animals from losing bone, a symptom of early osteoporosis.
This probiotic, renowned for its copious production of lactic acid, occurs naturally in some yogurts and other fermented dairy products. Bonus intestinal acid should increase the solubility of several minerals, including calcium, Schrezenmeir explains. Extra lactic acid should also spur the growth of cells lining the gut, he says, creating a bigger cadre to sop up released minerals.
To test these hypotheses, his group removed the ovaries from 6-month-old female rats. The ensuing drop in the rodents’ production of estrogen mimicked the hormonal environment of postmenopausal women. Over the next 16 weeks, the rats began losing bone, modeling what happens in many elderly women. However, calcium uptake from the diet was somewhat higher—and bone loss somewhat reduced—in animals given L. acidophilus.
Calcium uptake and bone mass improved even more when the researchers simply supplemented the animals’ diet with a material on which lactic acid bacteria prefer to feed. That supplement—known as a prebiotic—contained carbohydrates that only bacteria can digest.
Rodents receiving both prebiotics and probiotics retained the most bone and dietary calcium, the German team reported in the March 2007 Journal of Nutrition. Indeed, the combination restored bone mineral density and bone structure to about the level in rats with intact ovaries, Schrezenmeir says.
Tuning immunity
Probiotics are usually promoted as supporting intestinal health—a polite way of hinting that they may reduce the risk of diarrhea or bloating. Far less appreciated is the broad range of immune conditions for which they show promise.
The gut “is the body’s largest immune organ,” notes Arthur C. Ouwehand of the University of Turku, Finland, and of Danisco Innovation, a company that makes probiotics-enhanced foods. That’s why investigators at his and other research centers are exploring probiotics to improve immunity.
A study in 2005 by Schrezenmeir and his colleagues showed that daily treatment with a trio of probiotics didn’t reduce the incidence of colds. But the supplementation did reduce the severity and duration of cold symptoms—including fever—compared with a group of people that didn’t get probiotics.
“We don’t know the mechanism” for the probiotic advantage, Schrezenmeir says. However, in individuals given probiotics, the number of activated helper T cells—white blood cells that fight infection—increased, as did the number of germ-killing cells.
Probiotics may move the immune system in the opposite direction as well. Over the past year, several research teams reported some success with probiotics in treating inflammatory bowel disease. At least one study found they could help control exaggerated inflammation in intensive care patients at high risk for multiple organ dysfunction syndrome—a hyperinflammatory condition. And in a paper last August, Ouwehand recounted how probiotics administered to pregnant women and babies reduced the likelihood that high-risk infants developed food allergies.
In its newest work, Schrezenmeir’s team incubated immune cells from the blood of healthy or allergic individuals together with several immune-stimulating substances. Cells from all of the people responded, but only cells from allergic people showed an exaggerated response to allergens.
Adding four probiotic microbes or the naked DNA from probiotic bacteria to the mix substantially ratcheted down the response of immune cells, especially for people with allergies. About half of the immune-dampening effect in probiotic-treated cells was attributed to the live bugs, and half to their DNA—released when the beneficial bugs died. The work will appear in an upcoming Immunobiology.
Probiotic benefits are typically attributed to the fact that supplemented microbes were alive. However, receptors on the surfaces of both immune cells and cells lining the gut can bind DNA, Schrezenmeir notes. Probiotic DNA won’t be accessible to those cells until the microbe dies. His team’s new data suggest that probiotics—dead or alive—can affect systems in the body, perhaps by contributing to the communications among the gut’s native microbes.
Weight modulators
A number of food companies are investigating new health applications for probiotic supplements and fortified foods. Among novel functions being explored at the Nestlé research center in Lausanne, Switzerland, is probiotics’ control over calorie use.
Company scientists teamed up with researchers in England and Sweden for rodent experiments using strains of L. paracasei and L. rhamnosus, probiotics that Nestlé discovered years ago.
To create gut ecosystems in rats that model those of humans, the scientists seeded the guts of newborn mice—animals that were still germfree—with microbes from the digestive tracts of human babies. Beginning 6 weeks later, the researchers doctored the animals’ drinking water for 14 days with one or the other of the probiotics.
In the Jan. 15 Molecular Systems Biology, Nestlé biochemist Sunil Kochhar and his colleagues report that both strains of tested lactobacilli increased the hosts’ breakdown and use of simple carbohydrates. The data suggest that by helping people absorb more of the calories present in carbs, these or related probiotics might one day help fight malnutrition in parts of the world where carbohydrate-based diets are common, Kochhar says.
But probiotics can push this metabolic pendulum the other way.
Bile acids, produced mainly in the liver, play an important role in emulsifying dietary fats, a step that readies such lipids for digestion. The Nestlé probiotics broke down taurocholic acid, an especially efficient emulsifying bile acid. The resulting cholic acid “is not a good fat emulsifier,” notes Nicholson, a coauthor of the study—and after the probiotic treatment there was a 50-fold higher ratio of cholic to taurocholic acid in the treated animals’ guts.
This change diminished the rodents’ uptake of dietary fat and also reduced their synthesis of potentially harmful fatty substances in the blood, such as low-density lipoprotein cholesterol.
Where obesity is a problem, the same bugs might help people limit weight gain by diminishing their absorption of fats. “You only need to take in 20 to 30 more calories a day than you expend to make you fat in 2 or 3 years,” observes Nicholson. “What we’re interested in is looking for [probiotic] microbes that might help you absorb 50 calories less a day.”
These metabolic findings complement observations by Gordon’s team. The ecology of guts in lean and obese rodents is dominated by different bacteria, the Washington University researchers reported in 2006 in Nature (SN: 5/19/07, p. 314). The same holds for people.
After collectively identifying all of the microbial genes present in the guts of the naturally lean and obese mice, “we found that genes involved in breaking down otherwise indigestible complex carbohydrates were much better represented in the obese animals’ gut communities,” Gordon says.
His group then transplanted gut flora from a lean or obese mouse into a germfree animal and fed all treated rodents the same amounts. Animals that had received the gut microbes from obese animals gained more fat than did the animals given flora from a lean mouse.
Such experiments “show that differences in gut ecology influence the efficiency with which the bugs extract energy from foods,” Gordon says. However, his team’s data also show that gut microbes can alter what share of consumed energy will be stored as body fat.
Identifying the specific microbes responsible for these effects could point to new classes of weight-controlling probiotics, Gordon suspects.
Special effects
For all of their potential weight-modulating similarities, the two Nestlé probiotics had additional—and very different—actions. While the L. rhamnosus treatment dramatically decreased gut populations of potentially lethal bacteria known as Clostridium difficile (SN: 2/18/06, p. 104), the L. paracasei probiotic offered no defense against these germs.
There may be some direct effect of the probiotic microbes on these germs, or even on food metabolism, Nicholson says. But his new data suggest that many of the probiotics’ effects might best be characterized as microbial diplomacy—where small delegations of ingested germs persuade an army of resident microbes to adopt activities that better benefit their host.
“Bacteria talk to each other all of the time,” he says. Although there may be billions of local organisms, most “tend to behave like multicellular organisms,” he explains. These mega-beings coordinate their activity via microbial chatter. They signal their intent through the production and secretion of specific molecules.
“What we think is happening,” Nicholson says, “is that the probiotic bugs enter the gut, producing their chemical signals.” Relative to the hordes of microbes living in the gut, the incoming microbes make up only a teensy minority. However, based on the chemical dispatches issued during their transit through the intestines, the gut’s longtime residents “start to change what they’re doing.”
In the new study, Nicholson’s group showed that the messages relayed by each of the Nestlé probiotics seem to hit different families of resident flora, leading to different metabolic effects. One implication, he says, is that depending on which microbes permanently inhabit any particular individual’s gut, the probiotic’s message may resonate loudly or fall on deaf ears.
So which probiotic is most likely to work for an individual may depend on the precise nature of his or her flora, Nicholson maintains. The challenge, he says, will be to find out which flora are present and in what numbers. In a paper due out soon in the Proceedings of the National Academy of Sciences, his group will report the ability to get a rough inventory of those flora by analyzing their metabolic detritus in human urine.
Because of “the significant involvement of the gut microbiota in human health and disease,” gut flora might make good targets for medicines, Nicholson and his colleagues argue in the February Nature Reviews: Drug Discovery.
Consider that there are only about 3,000 human genes available to target with drug therapy—but “probably 100,000 gene targets in your gut microbiome,” Nicholson says.
To succeed, drug companies will need a better picture of the human gut’s microbial genome. It so happens that the National Institutes of Health recently established the Human Microbiome Project to nail that down.
Not without Risks
Probiotics exhibit a dark side
By design, probiotics should be helpful at best, benign at worst, notes Jeremy Nicholson of Imperial College, London. Side effects can occur, however, so unless people are battling an illness, he warns against consuming such microbes indiscriminately.
“If it ain’t broke,” he argues, “don’t fix it.”
The downside of probiotic therapy usually amounts to unexpected diarrhea. However, infections in the liver, heart, and other organs have also been linked to probiotics, according to a 2006 review by Robert J. Boyle of Royal Children’s Hospital in Victoria, Australia and his colleagues. Although the infectious agent in some cases was identical to the probiotic used, Boyle’s group notes that an indicted strain of microbe may sometimes also “be found in the internal microbiota of healthy humans, so the source of infection in these cases is not conclusively [due to probiotics].”
Last year, researchers reported in the September Pediatric Intensive Care Medicine that they had shut down a pediatric trial with Lactobacillus rhamnosus GG (LGG), a widely used probiotic, owing to growing concern that it might actually spawn infections.
Looking to cut the risk of hospital-acquired infections in severely ill children, Travis C.B. Honeycutt of WakeMed Health and Hospitals in Raleigh, N.C., and his team began randomly assigning kids to receive a probiotic or a placebo capsule daily while they were hospitalized in an intensive care unit. However, when three reports of LGG blood-borne infections in children emerged in quick succession from neighboring physicians outside the trial, the North Carolina researchers decided to perform an interim analysis to check whether LGG was as benign as they had told their patients’ parents it was.
“That analysis showed no benefit in our patients,” Honeycutt recalls, “and a trend—although it was not statistically significant—towards increasing infections in our probiotics group.”
But the really big wake-up call came last month, when Dutch researchers published findings of a trial using probiotics in people with acute pancreatitis. Patients provided nutrition laced with six probiotics experienced a death rate nearly triple that of people fed just the nutrients (SN: 2/23/08, p. 115).







