How patient-led research could speed up medical innovation
People with understudied chronic conditions are taking up science

Suffers of long COVID, ME/CFS and other chronic conditions are taking medical research into their own hands.
Pete Ryan
Melissa Red Hoffman was “feeling really stuck” last summer. A 50-year-old surgeon in Asheville, N.C., Hoffman had been struggling with long COVID since getting infected with the coronavirus two and a half years earlier. “Deafening fatigue” was one of her worst symptoms, she says. “I feel tired behind my eyes from the moment I get up to the moment I go to sleep.” She managed to work part time, but much of her work had shifted to administrative tasks that she did from her couch.
“I was really at a point where I had tried so many different things myself, with so many different providers,” she says, “not really sure what the hell to do next.”
Then she found Remission Biome. It’s a research project started in early 2023 by Tamara Romanuk and Tess Falor, two people with myalgic encephalomyelitis/chronic fatigue syndrome, or ME/CFS, a chronic disease that shares symptoms with long COVID. Project participants have taken medical research into their own hands to determine whether and how changes to their gut bacteria can improve their health. After an initial test with three participants led to some symptom relief, Romanuk and Falor announced last July that they would recruit 50 people with ME/CFS, long COVID or both for a larger test of the project’s protocol.
Hoffman was one of 500 people who applied within 36 hours of the call for volunteers. By the fall, she and 49 other people, dubbed the “Renegade 50,” had joined the project.
Remission Biome’s protocol is a multistep process, which participants undertake in consultation with their physicians. Initial steps involve patients collecting samples of their guts, immune systems and other connected organ systems, either at home or at a health care provider’s office. After those samples are analyzed by a lab to get baseline data, participants take a regimen of over-the-counter supplements, such as probiotics to cultivate certain types of gut bacteria, and then a prescribed antibiotic. Next comes further testing to examine if and how the regimen altered the composition of the gut microbiome. Throughout the process, participants track their symptoms and learn about past research on the microbiome that informed the project, ensuring that they understand the rationale for every step.
Early in the testing process, Hoffman’s fatigue started to lift, she says. “That’s been exciting, just to feel a little bit of a change.”
Alleviating symptoms — which can include debilitating fatigue, trouble sleeping, intense allergic reactions and cognitive problems — motivates many members of the Renegade 50, who come from different countries, age groups and stages of illness. But participants also aim to collect and publish data that will give the broader scientific community more information about ME/CFS and long COVID, two complex, often fluctuating conditions.
Participant María Richardson, a 36-year-old former educator in Mexico City, has dealt with progressively worse ME/CFS symptoms since high school. She received her diagnosis in the United States in 2015, but when she moved back to her native Mexico, where knowledge of the condition is limited, trying to get care “was like starting from zero,” she says. Remission Biome helped her better understand her own symptoms and share scientific information with the ME/CFS community in Mexico, through the ME/CFS advocacy group Millions Missing Mexico.
Remission Biome is one effort in the growing movement of patient-led research, which seeks to investigate chronic conditions that have been under-researched by academic and clinical scientists yet impact many people’s lives.
“People who were ignored by the American health care system … often need to turn to each other in order to gather the data that gets the attention of the mainstream,” says health care researcher Susannah Fox, author of the new book Rebel Health: A Field Guide to the Patient-Led Revolution in Medical Care.
Compared with mainstream medical research that tends to focus on finding biological causes and disease cures, patient-led work is more often rooted in what’s immediately relevant to patients’ daily lives, like identifying symptom triggers or relievers. But the approach faces challenges — particularly a lack of funding and other research resources — as scientific institutions aren’t set up to support these projects.
Patient-researchers and their scientist collaborators say the patient-led approach has big potential to move chronic disease research forward, making it more informed, quicker and more poised to directly improve patients’ lives.
Projects like Remission Biome “are going to change how research into these chronic, multi-organ-system diseases is going to be done,” Hoffman says. The approach may someday become a standard part of more mainstream research.
Experimental design
Remission Biome is studying whether changes to the gut microbiome can improve the health of people with ME/CFS or long COVID. Participants follow a protocol that includes taking prebiotic and probiotic supplements and a course of antibiotics.
Initial safety checks and testing: Samples from several body systems are collected and analyzed to ensure it’s safe for a volunteer to participate and to collect baseline data.
Symptom tracking: Participants record their symptoms throughout the project.
Start supplements: Participants begin taking prebiotics and probiotics.
Antibiotic added: An antibiotic is added to the regimen for 14 days.
Post-antibiotic testing: Body systems are checked again to look for changes.
Result: Remission Biome members hope to experience some symptom relief, as well as publish study findings and inspire new research projects for professional scientists.
A history of patient activism and patient-led research
About 1.3 percent of adults in the United States have ME/CFS, according to the U.S. Centers for Disease Control and Prevention. Scientists first noticed the condition in the 1930s, but since then, it’s been hard to define and hasn’t attracted extensive research attention. Initial observations noted outbreaks characterized by fatigue, chronic pain and other symptoms now associated with ME/CFS, often occurring — but not always — after viral infections. Scientists started to link these mysterious outbreaks in the 1980s under the umbrella term chronic fatigue syndrome.
Progress on identifying the disease’s triggers has been slow, in part because of the wide variety of symptoms across many organ systems and in part due to relatively limited research funding. And some doctors have dismissed patients’ symptoms as all psychological — a factor that some experts connect to the disease’s higher burden on women.
Combined, these challenges have contributed to a lack of treatments for people with ME/CFS, despite the illness’s potentially devastating impact on patients. Long COVID — which 6.8 percent of U.S. adults currently have, according to data from the CDC and U.S. Census Bureau — raised the profile of ME/CFS during the pandemic because of the two conditions’ similarities (SN: 3/4/24).
Susannah Fox
“Biomedical research has blind spots.”
Remission Biome started thanks to a Twitter conversation in fall 2022. Falor and Romanuk realized they had both independently experienced what they call “remission events,” in which symptoms recede for a few hours or days after courses of antibiotics. These events led each of them to look into the possible connection between ME/CFS symptoms and the gut microbiome, an emerging area of study with many unanswered questions. The pair were also both working scientists before their symptoms became debilitating. Falor had worked as an aerospace engineer at NASA; Romanuk had been a biologist studying microbiomes.
The two scientists set out to replicate their remission events — and collect extensive data on how their microbiomes and bodily systems changed to better understand the underlying biology of these events. They started with a self-test in early 2023, which included taking a lengthy list of supplements chosen to either increase or decrease levels of specific bacteria with possible ME/CFS connections. In addition to Romanuk and Falor, Isabel Ramirez-Burnett, a 50-year-old engineer and health coach in Rhode Island who has lived with ME/CFS since childhood, participated in the experiment.
The testing “went even better than we could have expected,” Falor says, with two of the three participants experiencing remission events. So Remission Biome expanded to the Renegade 50 cohort and fundraised through a crowdfunding campaign, grants and sponsorships to support this larger project. The team also recruited the participants’ physicians, to help ensure safety, along with scientists to collaborate with the participants and other volunteer researchers working on the project. Scientists regularly attend research meetings hosted by Remission Biome, Falor says, which include presentations and discussions about new, relevant findings in other ME/CFS and long COVID research.
Theoharis Theoharides is one of those scientists. As director of the Center of Excellence for Neuroinflammation Research at Nova Southeastern University in Clearwater, Fla., he has decades of experience studying mast cell activation syndrome, a chronic condition characterized by intense allergic reactions that is often diagnosed alongside ME/CFS and long COVID. “They’re very bright, very dedicated,” Theoharides says of Falor and Romanuk. He has provided feedback on Remission Biome’s regimen of supplements and plans to help analyze microbiome and blood samples taken from the Renegade 50 participants to look at how immune system changes may connect to their gut bacteria.
Another collaborator is Tatyana Dobreva, cofounder and CEO of the San Francisco–based biotech start-up ImYoo, which operates remote clinical trials and other research. ImYoo is assisting Remission Biome with genetic analysis of patient blood samples. The Renegade 50 study is similar to other ImYoo projects studying conditions such as inflammatory bowel disease and sickle cell disease, in which participants tie symptom tracking to data from medical testing, Dobreva says.
Remission Biome adds to a long history of patients with complex and contested illnesses advocating for their communities, Fox says. “Every decade of the 20th century had an example of people who were either being ignored or who were being discriminated against” by scientists and doctors, and who “banded together to innovate or gather data,” she says. Examples include Black people with sickle cell disease in the 1970s and people with HIV/AIDS in the 1980s (SN: 12/8/23). In some cases, this translated to patient-informed research, in which patients consult on scientific projects, informing everything from research questions to how results are disseminated.

In the 21st century, the internet aided patient-led projects, with patients actually doing research, as like-minded patients could more easily find each other, as happened with Romanuk and Falor, Fox says. In these projects, patients also closely follow scientific studies about their disease and may collaborate with academic experts to develop scientific frameworks, rather than self-experimenting individually.
ME/CFS patients have been particularly motivated to pursue their own research, says Emily Taylor, vice president of advocacy and engagement at the ME/CFS organization Solve M.E. One key motivator is “the failure of the medical establishment to provide any sort of support or treatment or quality of life improvements for this population,” she says. Previous ME/CFS research done without patient input, such as a now-debunked clinical trial examining exercise as a potential treatment, has led patients to push back with their own studies.
“There was a desperate need to validate the anecdotal stories of patients in a formalized way,” Taylor says.
In spring 2020, during the first months of the pandemic, patients whose symptoms persisted for weeks after the initial infection started documenting their complex symptoms in real time. The Patient-Led Research Collaborative, or PLRC, formed out of a long COVID support group, led by members who had scientific experience.
PLRC released its first report in May 2020, documenting symptoms common among the group’s hundreds of members. “We saw a need to start collecting people’s experiences and really try to take things into our own hands,” says PLRC cofounder and long COVID patient Lisa McCorkell.
1.3
percent
U.S. adults who have ME/CFS
6.8
percent
U.S. adults who have long COVID
Patients are experts
Patient-led and patient-informed research can be a win-win for both patients and scientists, advocates say. For patients, this work is more likely to address questions that are meaningful to their daily lives, says Jaime Seltzer, director of science and medical outreach at the advocacy group #MEAction. In one pre-pandemic example, a patient group focused on polycystic kidney disease proposed potential treatments to scientists at the University of Cambridge, leading to clinical trials at a new patient-led research hub.
Patient leadership can also inspire people to participate in clinical trials, as the interest in joining Remission Biome demonstrates. And study designs informed by patient experience often prioritize accommodations for people with different levels of symptoms or access to care, meaning a more diverse group of patients may be able to participate. With a patient-led, “decentralized” approach to research, “we can reach more people in more diverse areas” who don’t live near medical facilities in big cities or aren’t able to travel for clinical trials, Dobreva says.
Connor, a member of the Renegade 50 who asked that only his first name be used to maintain medical privacy, “couldn’t participate in a traditional study,” says his wife, Nicole Bruno. Since a COVID-19 infection two and a half years ago, he has faced a severe case of both long COVID and ME/CFS, leaving him bedbound in a dark room.
“He could never go to a lab” or a doctor’s office to have samples collected, Bruno says. But with Remission Biome’s remote framework and individual support, he can be a patient-researcher. In addition to flexibility in locations, each member of the cohort is going through the testing protocol at their own pace, incorporating their microbiome test results, other diagnoses and input from their physicians. Flexibility also helps with logistical challenges; for example, test kits take longer to ship internationally.
For scientists, patient-led studies may move a field forward by highlighting key questions and hypotheses that might not emerge from traditional research. “Biomedical research has blind spots,” Fox says. McCorkell points to a paper from the PLRC, published in eClinicalMedicine in 2021, that expanded upon its 2020 survey work by describing 200 long COVID symptoms across 10 organ systems based on a detailed survey of about 3,800 people.
“It is still, to this day, one of the most cited papers in long COVID,” McCorkell says. Without this paper, she adds, other scientists might still be investigating “a small, limited set of symptoms” rather than the full scope of the condition. David Putrino, a long COVID clinician and director of rehabilitation innovation at Mount Sinai Health System in New York City, also points to the PLRC paper as an example of successful patient-led research that informed later studies.
Patient-led research “moves orders of magnitude faster than traditional modes of research,” Putrino says, because it focuses on the questions that are of greatest concern to patients, leading more quickly to impactful results. Patient-led groups may also be able to start new studies more quickly than institutions that have to, say, go through formal academic procedures, he says. In that way, this research is similar to how start-ups move faster than large corporations.
In addition, patients can help scientists design studies that are more likely to provide accurate results. For example, feedback from members of Remission Biome and other patient representatives helped David Esteban, a biologist at Vassar College in Poughkeepsie, N.Y., who was looking for people who had gotten COVID-19 but didn’t develop long COVID and could serve as control patients in a project funded by PLRC.
“Their perspective was, many people who recover from acute COVID go through a period where they feel better, but then get worse again,” he says. “I hadn’t really thought about that.” But that insight helped Esteban establish how long after a COVID-19 infection to wait before declaring a patient past the threshold for developing long COVID.
After studies are completed, patient teams may be more thoughtful about communicating results back to patient communities. In sharing a recent paper about managing ME/CFS that she coauthored with clinicians at the Mayo Clinic in Rochester, Minn., for instance, Seltzer anticipated questions that ME/CFS patients might have about the study. She explained up front that the paper was a concise review and could not include every relevant study, as patients would want to know “why I hadn’t mentioned their favorite paper,” she says. Such communication can “save a research group a lot of time and energy,” Seltzer says, and can encourage patients to bring the paper to their doctors so that the findings might inform their health care.
Groups like PLRC are working to build infrastructure to help scientists better engage with patients, including experienced patient-researchers and others who haven’t done scientific work before.
In January 2023, PLRC and the Council of Medical Specialty Societies introduced scorecards for academic teams interested in these collaborations. The scorecards can help teams evaluate success. “Our scorecards were developed with the intention of trying to change the baseline of what’s considered acceptable patient engagement,” McCorkell says, moving away from “tokenizing” engagement that she and other PLRC members have experienced. Taylor, at Solve M.E., would like to see the scorecards or a similar evaluation incorporated into traditional funding applications at scientific institutions.
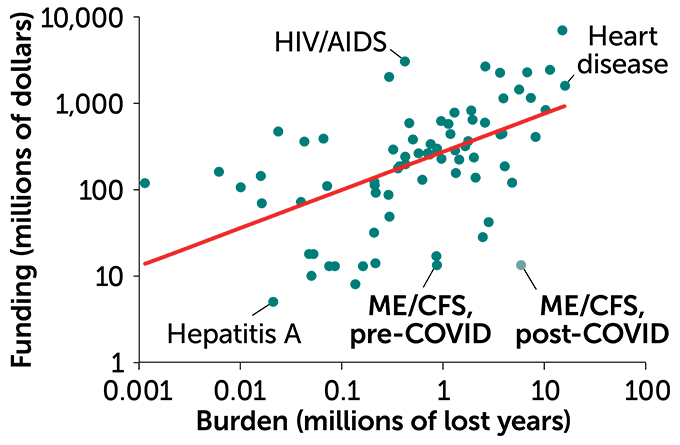
ME/CFS funding
Of 73 diseases (dots) with research funding from the National Institutes of Health, ME/CFS is among those underfunded relative to its disease burden, the total number of healthy years lost due to premature death or disability from illness. The graph includes an estimate of ME/CFS burden with the arrival of COVID-19. The red line is expected funding levels based on burden.
NIH funding vs. disease burden, 2020
The challenges of patient-led research
Current institutional and financial support for patient-led research projects is limited. These projects typically are not eligible to apply for academic and government grants, leading them to seek money from nontraditional sources. Patient-researchers also don’t tend to have access to laboratory space, clinical tests and other research resources.
“We’re limited in the type of research that we can do,” McCorkell says. As a result, surveys and self-experimentation are the most common methods.
Internal capacity is another challenge: Chronically ill people tend to have limited energy to devote to projects; they must balance this work with managing their symptoms. Patients tend to be more ambitious than their available energy can support, Seltzer says. Sometimes a patient-researcher might have to take a break from a project to recover from a symptom flare-up. Projects like Remission Biome take these crashes into account when designing experiments and distributing tasks.
“If I disappear for a week,” it’s OK, says Katrin Boniface, a doctoral student studying the history of horses at the University of California, Riverside who had her own remission experience before joining the Renegade 50. But these constraints might frustrate academic or clinician collaborators who want patient-researchers to answer emails at all hours or pull together a last-minute grant proposal.
Nonpatient scientists might also be skeptical of results from patient-led research, as many in the scientific community haven’t yet recognized how lived experience can improve studies, Seltzer says. Although many patient-researchers have scientific backgrounds, they might not be experienced in biomedical research, leading to perceptions that they are underqualified and that their work is not rigorous or may even be biased.
Advocates like Seltzer argue that patient-researchers are more incentivized than anyone to make sure their results are accurate. “If we’re wrong, we and people like us suffer,” she says.
Taylor argues that data from patient-led research should be added to the types of evidence that regulatory agencies like the U.S. Food and Drug Administration consider for approving treatments. The FDA and the National Institutes of Health took one step in this direction earlier this year by soliciting data from long COVID patients and doctors about their experiences with treatments approved for other diseases.
“There was a desperate need to validate the anecdotal stories of patients in a formalized way.”
Emily Taylor
But some scientists and doctors are concerned that patient-led projects might encourage some patients to self-experiment on their own without appropriate safety measures. This has been a big challenge for Remission Biome, especially after its members posted about remission events during the project’s first phase in early 2023. Initially, the plan was to openly share all aspects of the project, including protocols and results, says Ramirez-Burnett, one of the three early participants. “But then we realized that people were starting to pick pieces of the protocol in order to do it, which is not safe,” she says. “So we had to close that document.”
Now, when asked about the full protocol, as they often are on social media, Remission Biome participants typically encourage safety and emphasize that more testing is needed before it’s widely shared. In the future, Ramirez-Burnett hopes to educate more clinicians about the project so they can work with patients outside the Remission Biome infrastructure.
Patient-led projects may also struggle with logistics. This has been the case for Remission Biome. Its two founders split in December over disagreements about the project’s pace, its handling of safety aspects and how to incorporate the project as a formal business. As a result, Romanuk and the group parted ways.
The Renegade 50 test was put on hold until mid-March while Falor and other project members addressed this leadership change and set up as a nonprofit, she says. The team is also adding more safety steps and participant education on the antibiotic in the testing protocol because that antibiotic may have negative side effects for some people with ME/CFS. Falor expects the Renegade 50 phase will be completed later this year, after which the project will share preliminary results and begin setting up a cohort of 500 participants.
Tests and supplements for that next cohort will require more financial support, which Remission Biome will continue to raise from its GoFundMe campaign and grants. The project has also secured sponsorships from supplement and testing companies, such as the probiotics provider FitBiomics, to provide research supplies to participants. Financial support is especially important for participants living in places where it’s difficult to receive medical care for ME/CFS, says Richardson, the Renegade 50 member in Mexico. Many patients globally could benefit from this work, she says.
Remission Biome is also working toward scientific publications, based on data from the Renegade 50 cohort and from side projects. But the 50-person test might not lead to publishable results, says scientist-collaborator Theoharides. The microbiome is extremely complex, and, unlike a clinical trial, the Renegade 50 group does not include control patients not taking the treatments. But he hopes “the information that will come out of this study might actually give us some new directions.” One key advantage, he says, is that each participant is testing many supplements rather than focusing on one at a time; ME/CFS and long COVID are such complex diseases that it’s unlikely for a single treatment to work for all patients or have a lasting impact.
Esteban, the biologist at Vassar College, similarly hopes to examine how different antibiotics might work together to alleviate symptoms. “I’m already thinking about experiments that I could do,” he says, such as work in lab animals that would “start to explore some of the proposed mechanisms that might underlie the effects they’re seeing with the antibiotic treatments.”
While Remission Biome’s participants are excited to contribute to research, their most important goal is to provide “solutions for the ME/CFS community,” Ramirez-Burnett says. “So people don’t have to lose their jobs, lose their relationships, not get proper care.”
Among the three Renegade 50 participants who had completed the testing protocol as of January, one experienced a remission event, signifying a potential success, Falor says. Meanwhile, the project’s frequent meetings, Slack group, apps for shared symptom-tracking and other communication options could provide models for other patient-research efforts.
Remission Biome participants who have dealt with ME/CFS for a long time, like Richardson, feel particularly motivated to help find answers for the millions around the world newly struggling with long COVID. “People with mild long COVID sound like what I experienced 20 years ago,” Richardson says. She hopes that the lessons learned from Remission Biome and other projects like it can help prevent new long COVID patients from experiencing decades of symptoms.







