150 years on, the periodic table has more stories than it has elements
Its organization holds stories of discovery and strange reactions

INTRINSIC ORDER Every element on this venerated table has its own story. All together, they capture the entire repertoire of known chemistry.
E. Otwell
Recognize these rows and columns? You may remember a detail or two about this mighty table’s organization from a long-ago chemistry class. Elements are ordered according to their number of protons, or atomic number. Metals are mostly to the left and nonmetals to the right. The column at the far right holds the noble gases, named for their general unwillingness to interact with other elements.
When Dmitrii Mendeleev proposed his periodic table 150 years ago, no one knew what was inside an atom. Today, we know that an element’s place on the table, along with its chemical properties, has a lot to do with the element’s proton number as well as how its electrons are configured.
In one glance, you can see the elements that make up nature’s entire
repertoire of chemical substances plus how those elements relate to one another. But the elements are also individuals, with scientific idiosyncrasies and nuanced stories of discovery. A few of our favorites are on these pages.
And the table is still a work in progress. Four elements were named as recently as 2016. Boundary-busting research efforts, along with scientific mysteries, remain.
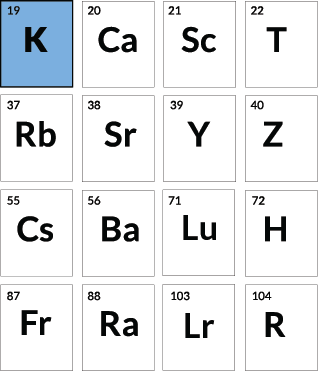
Banana bonanza
Potassium
Bananas are rich in potassium-40, a radioactive version of potassium. In a single banana, the potassium-40 produces a positron, the antimatter version of the electron, a dozen or so times a day, as well as an electron about 13 times a second.
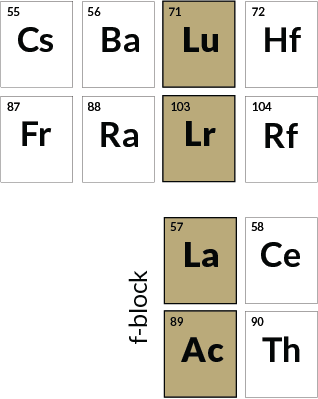
Who belongs
Lutetium, Lawrencium, Lanthanum, Actinium
Not everyone is convinced that lutetium and lawrencium belong in the upper positions shown here. The Royal Society of Chemistry instead puts lanthanum and actinium in these upper boxes, prioritizing outer electron configurations and sticking lutetium and lawrencium at the end of the f-block. The International Union of Pure and Applied Chemistry, responsible for chemical naming, has been exploring the placement question since 2015.

Out of the lab
Uranium
When Henri Becquerel, a French physicist, placed uranium salts atop photographic plates in 1896, he accidentally discovered radioactivity, for which he won the Nobel Prize in physics in 1903. Uranium is the last element on the table that occurs in any meaningful abundance in nature; the rest must be created in the lab.
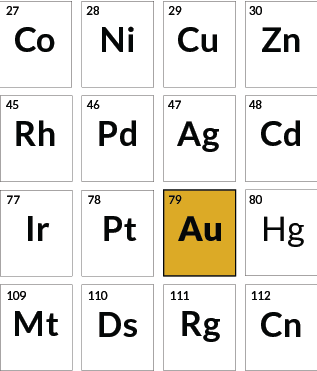
Special luster
Gold
Albert Einstein’s special theory of relativity explains gold’s color. Because of how electron energy levels shift due to relativity, the metal absorbs blue light, giving the reflected light a yellow hue.
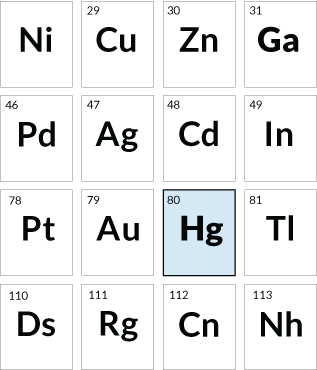
Campsite clues
Mercury
When Meriwether Lewis and William Clark set out to reach the Pacific Ocean, they carried 1,300 doses of a mercury-based laxative known as Rush’s Thunderbolts. Mercury discovered in the ground in Lolo, Mont., nearly two centuries later clued experts in to the location of one of the explorers’ campsites.
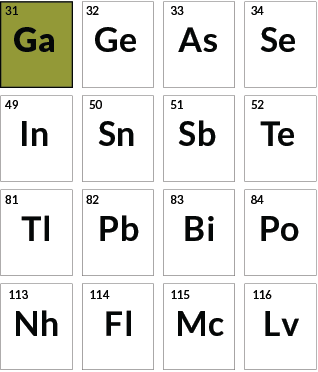
Predictive power
Gallium
Mendeleev left blank spaces in his original periodic table so that he could properly line up the known elements. Gallium, element 31, was his first gap to be filled, in 1875. The star of a popular chemistry trick, the metal gallium is solid at room temperature but liquid above 29.7° Celsius. It can be formed into a spoon that melts in the hand or in hot tea.
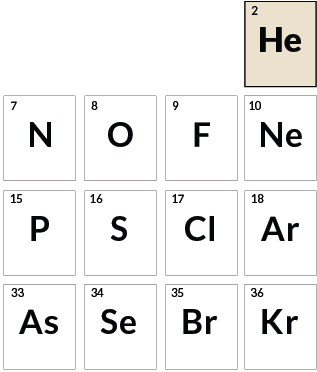
Out there
Helium
Helium was discovered as a bright yellow line in a spectrum of light from the sun in 1868, almost three decades before the element was found on Earth. Last year, scientists reported the first sighting of helium in the atmosphere of an exoplanet.

Three of a kind
Chlorine, Bromine, Iodine
Chlorine, bromine and iodine make up what German chemist Johann Wolfgang Döbereiner called a “triad.” Bromine’s atomic weight of 79.90 is halfway between chlorine’s (35.45) and iodine’s (126.90), and all react readily with metals to form salts. Döbereiner recognized such relationships in 1817, more than a half century before Mendeleev proposed his table.
The end?
Oganesson
Oganesson marks the end of today’s periodic table, capping the noble gases column. Yet it isn’t as aloof as others in its group. The element will readily give or take electrons, and its atoms may clump together — at least according to theoretical predictions. The few atoms of oganesson that chemists have made survived for less than a millisecond. Scientists continue smashing atoms together in the lab in search of elements beyond 118.