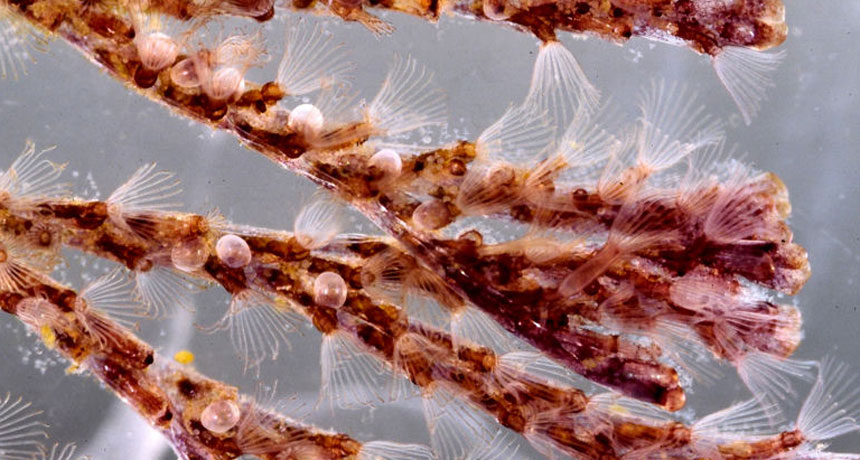
MARINE MEDICINE A bryozoan called Bugula neritina, shown here, is a natural source of bryostatin 1, which has been studied as a potential drug for many years.
Lovell and Libby Langstroth © California Academy of Sciences
A seaweed-like marine invertebrate contains a molecule that has piqued interest as a drug but is in short supply: Collecting 14 tons of the critters, a type of bryozoan, yields just 18 grams of the potential medicine. Now, an efficient lab recipe might make bryostatin 1 easier to get.
Making more of the molecule could help scientists figure out whether the drug — which has shown mixed results in limited clinical trials for cancer, HIV and Alzheimer’s disease — will pan out or bomb.
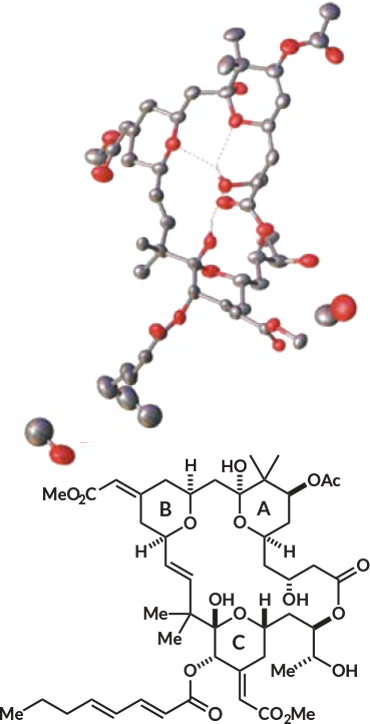
Bryostatin 1, found naturally in a sea creature called Bugula neritina, has been studied as a potential drug for several decades. It interacts with an enzyme in the human body that helps regulate cell growth and control immune response. But finding a way to re-create the molecule in the lab, which would ensure a steady supply for research, has been a challenge. It’s a large, unwieldy molecule with a complex structure — multiple rings and lots of appendages with different chemical properties.
The new recipe, reported in the Oct. 13 Science, has so far produced more than 2 grams of bryostatin 1.
It’s a big achievement, says Karl Hale, a chemist at Queen’s University Belfast in Northern Ireland who wasn’t part of the study. Other scientists have created bryostatin 1, but only milligrams of it. The several grams produced by the new recipe is enough to supply numerous clinical trials, says study coauthor Paul Wender, a chemist at Stanford University.
To build such a clunky molecule from simple chemical ingredients, Wender’s team fashioned the molecule in two parts through a series of chemical reactions, then linked the two together. The general reaction that links the two pieces together into one big molecule has been used before by other chemists working to synthesize bryostatin 1 and related compounds. But Wender says that his team’s approach works in a slightly different way.
The whole process involved a lot of atom shuffling: adding on and removing different appendages, for instance, and forming and then breaking rings that would never make it into the final product. In total, the process takes 29 different chemical reactions — an improvement on the 57 steps previously reported because with each step, some material is lost.
Other bryostatins produced by B. neritina have similarly intricate structures with slightly different appendages, and also might have medical use. Scientists have re-created some of those in the lab, too, some through processes almost as streamlined as this one. But bryostatin 1 was important to make because it’s the only version of the molecule that has been used in clinical trials, Wender says. The process can also be tweaked to create bryostatin-like molecules that aren’t found in nature, but that might work better as drugs, he says.
But not everyone is convinced that bryostatin 1 and its chemical relatives are still worth pursuing as a drug. Investigated as a cancer treatment for many years, the molecule didn’t turn out to be very effective against tumors, Hale says. And many patients reported severe muscle pain at the doses given for cancer therapy.
Wender is an adviser for a company called Neurotrope BioScience that’s developing bryostatins and related molecules to treat Alzheimer’s and other neurodegenerative diseases. Results released earlier this year from a clinical trial in mid- to late-stage Alzheimer’s patients were “a bit of a mixed bag,” says Hale. Some patients on the lower dose of the drug saw some improvements on a cognitive test over those who got the placebo, but the small differences could have been due to chance.
Giving the drug at a different dose, or treating patients for longer or at an earlier stage of Alzheimer’s might yield better results, but “the jury is still out,” Hale says.
Wender and others are also pursuing clinical trials for bryostatin 1 as a drug for HIV. An early-stage clinical trial published in 2016 showed that the drug was safe but didn’t show any benefit against HIV at the low dose used. Expanding access to the compound should help speed up this sort of research.