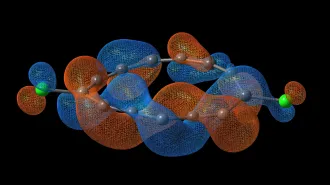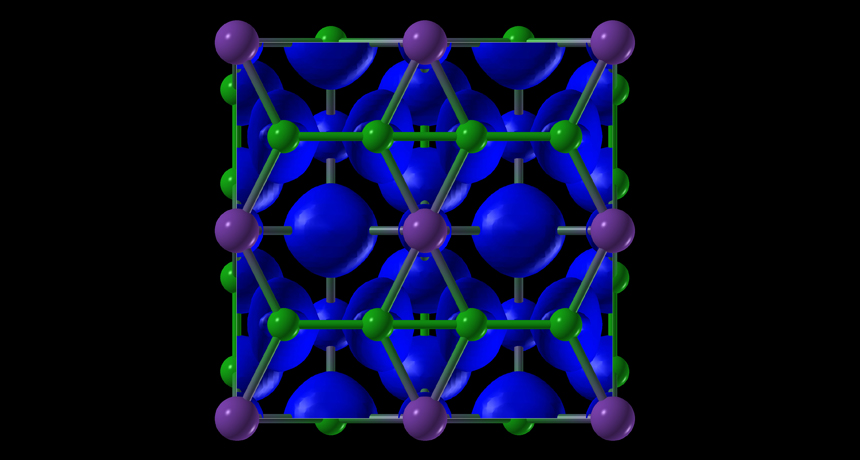
SALT GONE WILD Experiments using extreme conditions reveal that common table salt, NaCl, can form exotic compounds, such as NaCl3. In this illustration, purple represents sodium, green represents chlorine and blue represents electron clouds.
Courtesy of A.R. Oganov and Weiwei Zhang
With a dash of table salt, scientists have created exotic compounds that bend the basic rules of chemical bonds and matter in the universe, researchers report in the Dec. 20 Science.
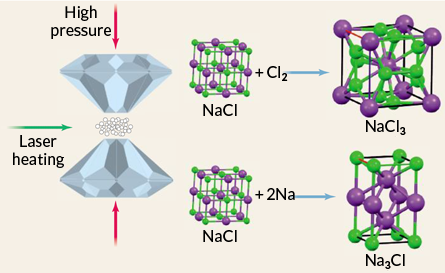
The team pulled off the spicy chemistry by squeezing grains of sodium chloride between two diamonds while frying the salt with a laser.
The high-temperature, high-pressure conditions mimic those inside stars and planets, says chemist Artem Oganov of Stony Brook University. His team’s discovery that these conditions create unexpected chemicals suggests that alien chemical structures make up a vast fraction of the solar system’s matter.
“This is a new chapter of chemistry,” Oganov says. It means that the rules in chemistry textbooks have much more limited applicability than previously thought, he says.
Scientists had predicted that extreme conditions could change how atoms interact and bind together. For instance, chemists expected that high pressure could convert ionic bonds such as those in salt, in which one atom donates an electron to another atom, to metallic bonds, in which electrons travel more freely among atoms.
But Oganov and his colleagues went beyond unconventional bonds to discover entirely new compounds, says physicist Jordi Ibáñez Insa of the Institute of Earth Sciences Jaume Almera in Barcelona. This is the first experimental evidence of exotic compounds ordinarily not found on Earth, Insa says.
At Earth’s surface, sodium and chlorine atoms normally bind only in a 1-to-1 ratio to make NaCl, which has an internal structure with atoms arrayed in neat cubes. But in the hot, pressurized experiments, Oganov and his colleagues found that sodium and chlorine could also bind in novel ratios such as 1-to-3, 3-to-2 and 1-to-7.
By using X-ray diffraction measurements, the researchers discovered that the extreme conditions altered how the atoms bonded by allowing them to get closer to one another. In doing so, the compounds break the so-called octet rule, which states that atoms in the first rows of the periodic table tend to form bonds that complete a set of eight electrons in the outskirts of their electron cloud. In the new compounds, the sodium and chlorine atoms did not always have eight electrons spinning in their outer orbits.
The new compounds of sodium and chlorine also formed rectangular and gemlike internal structures that had different properties. For instance, the compound NaCl3 probably conducts electricity, the authors predict based on computer simulations.
On a practical level, the study demonstrates how scientists might re-create extreme conditions to build novel and useful materials that violate conventional wisdom, says materials scientist Yanming Ma of Jilin University in Changchun in China.
Eugene Gregoryanz of the University of Edinburgh agrees. But so far, he notes, the newly created compounds don’t survive when they’re brought back to low-pressure, low-temperature conditions. “The jaw-dropping discovery,” he says, would be bringing the exotic compounds back to ambient conditions. In other words, if chemists made metallic table salt.
Editor’s Note: This story was updated on December 20, 2013, to clarify the description of ionic bonds.