Unsolved drugs
A simmering controversy surrounds what’s still unknown about how antibiotics kill
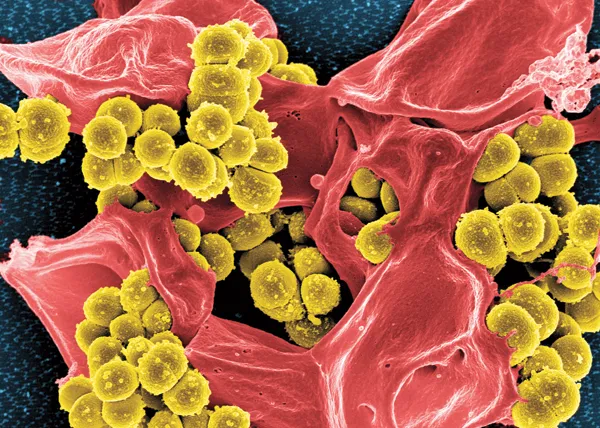
BATTLING BACK Antibiotics such as penicillin attack a bacterium’s cell wall, bursting it (left, shown next to an intact Staphylococcus aureus cell in false color). Some studies suggest that antibiotics also trigger other kinds of damage that could be harnessed in the battle against antibiotic resistance.
CNRI/Science Source
Penicillin attacks with a calculated strike, splitting open cell walls. Kanamycin sends a bacterium’s protein assembly line into mayhem. Ciprofloxacin dices a microbe’s DNA into a genetic hash. Like trained snipers, each of these common antibiotics seems to dispatch bacteria with a simple tactic: Target a high-profile molecule crucial to survival and, with a single, clean shot, defeat the whole cell.
For decades, this notion of how antibiotics kill has guided the design and deployment of drugs that have saved countless lives. Since scientists introduced penicillin in the 1940s, antibiotics have tamed some of the most deadly microbes into mild nuisances. Bacterial infections that cause tuberculosis, pneumonia and diarrhea, the three leading killers in the United States a century ago, have become curable or rare in the developed world.
But after myriad clinical victories, some scientists are questioning what we really know about the modus operandi of these bacterial assassins. In 2007, researchers proposed that, in addition to direct hits, antibiotics rev up cellular power plants and generate explosive waste. The resulting biochemical chaos might be as important to an antibiotic’s deadly force as the better-studied precision tactics. The intriguing idea of antibiotics as sloppy killers created a buzz followed by a heated controversy in an otherwise sedate corner of science, says Gerry Wright, who studies antibiotic resistance at McMaster University in Hamilton, Canada.
A string of studies have come out for and against the theory, with more under way. Last year, two critical studies sparked widespread skepticism of the science behind the theory. But the clash hasn’t deterred some scientists from digging further, in hopes that the fresh perspective could lead to new therapies.
Chief among them is systems biologist James Collins of Boston University, who first proposed the controversial theory. Most scientists, he says, thought that they understood how antibiotics kill. But scientists may know only a “tiny, narrow slice of what’s happening,” he says.
That might not be bad news: Understanding these unseen complexities of how drugs actually kill could inspire a new generation of desperately needed treatments as mounting numbers of bacteria become resistant to antibiotics.
In an evolutionary arms race with drug development, some microbes have evolved to guard or disguise prominent drug targets. Other bacteria have come up with ways of simply deporting drugs from their cellular borders. Health experts around the world have warned that modern medicine is losing a war that most thought was already won. In the United States, about 2 million people get sick with a drug-resistant bacterial infection each year and 23,000 or more die. As those numbers rise, bacteria keep developing new defenses. Some tuberculosis infections, for instance, can withstand assaults by every drug in a physician’s arsenal.
In response, researchers are busily drawing up blueprints for new antibiotics and picking out new molecular targets to attack. But the efforts have been slow, scientifically challenging and expensive. Between 1998 and 2012, the U.S. Food and Drug Administration approved 14 new antibiotics. Only four had novel mechanisms.
New thinking about how old drugs actually kill could speed the development of new therapies, says Collins. If collateral damage is important in killing microbes, then figuring out ways to increase that damage could help extend the usefulness of existing drugs. Collins envisions antibiotic sidekicks that would stir up molecular trouble in the cell, creating a combination therapy that would make existing antibiotics more lethal.
The idea is to restore the effectiveness of current antibiotics as opposed to discovering a new class of antibiotics, says Jeff Wager, president of EnBiotix, a Boston company developing drugs based on Collins’ work. “This just seems like a smarter, faster, more broadly applicable way to approach the problem,” he says.
But Collins’ antibiotic theory has vocal foes. “You never say never,” says microbial physiologist James Imlay of the University of Illinois at Urbana-Champaign. But to Imlay, the evidence against the idea is “as ironclad as you can get.”
Whoever is correct, the disagreement is telling of the many unknowns about how classic antibiotics work. It points to uncharted territory potentially rife with new targets and attack strategies. “I’m not convinced we really understand how all these antibiotics work,” says Wright. “The people who are trying to figure it out are doing us all a favor.”
Antibiotic aim
Collins, a biomedical engineer, didn’t set out to design new antibiotic therapies. In the early 2000s, he waded into the nascent field of systems biology. The field, which itself has created a stir among biologists, eschews traditional molecular techniques and instead applies an engineer’s logic to understanding how networks of proteins and molecules orchestrate the workings of an organism. When a graduate student came up with data revealing unseen effects of quinolone antibiotics, a class that treats skin, lung and urinary tract infections, Collins grew intrigued.
Generally, scientists have thought that quinolones such as ciprofloxacin and nalidixic acid kill bacteria by leaving DNA in bits. The drugs latch onto bacterial enzymes called topoisomerases, including gyrase, which slide along DNA strands, untwisting and unhooking the A’s, G’s, C’s and T’s so other molecular machinery can move in and repair, decode or make copies of the genetic sequence. Quinolones lock on after the enzymes break strands of DNA for untwisting, leaving dangling fragments. Since dozens if not hundreds of gyrase and topoisomerase enzymes work along a bacterium’s DNA at once, a large-scale invasion of quinolone molecules can swiftly reduce a whole chromosome to rubble. If the bacterium can’t copy its genes, it can’t function. The minced genetic mess, it seemed, spelled eventual death for the cell.
But Karl Drlica, a molecular biologist at Rutgers’ New Jersey Medical School in Newark who has studied the drugs for years, points out that damaged DNA does not necessarily equal cell death. “You get these drug-DNA complexes, and that blocks growth,” says Drlica. But bacteria have a repair system, aptly called the SOS response, to fix DNA breaks. Quinolones’ main strike “is reversible, so it’s not lethal, because death is not reversible,” he says.
Approaching the issue at a systems level, Collins and his team used tools such as DNA microarrays to take a cellular census of which genes were turned up or down during an antibiotic strike. “What our systems analysis shows is there’s more to the story,” says Collins. While scrambling to repair DNA, the microbes were also fending off cell-wide damage. Dying cells cranked up their metabolism, deployed fighters to mop up dangerous superoxide molecules and threw iron regulation into disarray. Unknown to scientists, quinolones triggered a bevy of defensive maneuvers.
Drugs of mass destruction
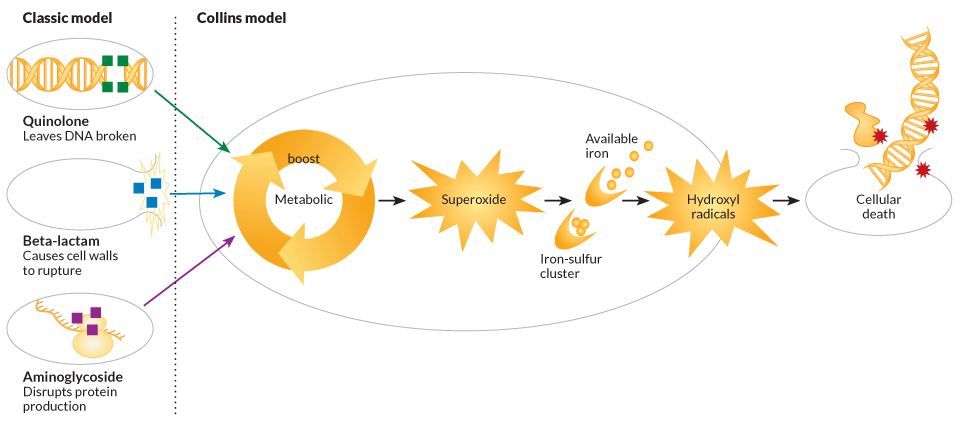
By investigating each defense separately, Collins and his team came up with a model of how it all fits together: The direct hit to DNA indirectly sends metabolism into overdrive as the cell desperately attempts to power up its defenses. The boost of activity at the bacterial power plants leads to a buildup of a cellular toxic waste product called superoxide. If there’s too much, it can overload a bacterium’s normal disposal systems. Spilling through a cell, the reactive molecule sets off its own cascade of collateral damage.
Superoxide, a type of reactive oxygen species made of two oxygen atoms, can blast open clusters of iron and sulfur found on certain proteins. Chemically available iron can mix with hydrogen peroxide, another noxious scrap of power production, to create hydroxyl radicals, a potent reactive oxygen species. These dreaded atomic bandits violently raid the cell, indiscriminately hacking electrons off molecules and wrecking DNA, cell membranes and protein-production machinery. The rampaging radicals can quickly destroy the whole cell.
To see if reactive oxygen species are generally involved in antibiotic killing sprees, Collins and his team turned to the two other classes of deadly drugs. One is the aminoglycosides, represented in the studies by kanamycin, which target the cellular equipment that translates RNA messages into new proteins. The team also looked at beta-lactam antibiotics, such as penicillin and ampicillin, which hack apart links in bacterial cell walls.
To monitor for antibiotic aftereffects, Collins and his colleagues set a flashy bait in bacterial cells — a molecule that glows when cut by hydroxyl radicals. In each case, the antibiotics triggered a flare of fluorescence, indicating a cascade of damage similar to that set off by the quinolones.
Next, the team sprinkled in another chemical, called thiourea, which can quell rebellious reactive oxygen species. With the chemical addition, researchers stifled the fluorescent flash, fueling the hypothesis of savage side effects. Plumbing deeper into dying bacteria, Collins and his team found more genetic and molecular evidence of off-target impacts. They monitored a coenzyme called nicotinamide adenine dinucleotide, which is crucial to cellular energy production, and found its activity boosted. Genes that control protein repair, such as hslU, and systems of DNA repair, such as the SOS response, were engaged. Mutant bacteria with no functional SOS response were more vulnerable to the three classes of antibiotics studied.
In 2007 in Cell and in 2010 in Nature Reviews Microbiology, Collins and his colleagues laid out a generalized model of how these classes of antibiotics set off a chain reaction leading to reactive oxygen species and death. Collins is careful to point out that the model isn’t suggesting an overhaul to everything scientists know about antibiotics, nor is it saying that reactive oxygen species are solely killing the cells. “We’re not overturning, but expanding upon,” accepted thinking, he says.
Opposition forces
That cautionary stance comes after a spate of fiery criticism. Last year in Science, back-to-back papers blasted Collins’ model, rejecting his experimental design and pointing out alternative interpretations of the data.
Imlay, lead author of one critical paper and an expert on reactive oxygen species, raised an eyebrow to Collins’ unconventional systems approach to investigating antibiotic assassinations. “It’s a great idea,” he says. But the theory that hydroxyl radicals play a role doesn’t hold up, he says. “If it were true, it would reveal something new about how reactive oxygen species would be made.”
In Collins’ model, the cell’s power plants kick off the cascade with spewed waste. But Imlay argues that energy production, even when cranked up, is a wimpy starter for the full-scale hydroxyl radical assault needed to kill off a cell. It usually makes only a small amount, he explains.
Story continues below diagram
With a phone call to Collins’ lab, Imlay’s group got the protocol for some of the experiments. The team could repeat the results, but found contrary data as well. The chemical bait that glows when cleaved by reactive oxygen species is not that specific. It can be hacked by other molecules produced during antibiotic death, Imlay says. When his researchers looked for a boost in free iron, central to the creation of hydroxyl radicals, they couldn’t detect it. “That’s the key reaction,” Imlay says. “And we measured it and did not see a change.”
Despite Collins’ finding that certain emergency repair systems spring into action during an antibiotic siege, Imlay points out that OxyR, a hallmark system for repairing damage from reactive oxygen species, was missing. And, when Imlay and coauthor Yuanyuan Liu looked at antibiotic attacks in the absence of oxygen, which is essential for forming damaging oxygen species, they found that the drugs were still lethal. The finding suggests that oxygen species aren’t required for — though they still could play a role in — death.
A second Science paper, coauthored by biologist Iris Keren, a former member of Collins’ lab, reexamined each experiment in Collins’ 2007 original paper in detail, pointing out flaws. Keren, who has since left academic research, did not respond to interview requests.
Though Keren’s personal connection to Collins has led many in the field to question the motivation of her work, the data have taken a toll on the reactive oxygen theory. “There is, I think, a fairly widespread perception that the overall Collins model has been disproven,” says molecular biologist Graham Walker of MIT, who has found supporting data for Collins’ model. Many doubt that it’s a universal explanation of how antibiotics kill.
But research continues. A battery of studies, including Walker’s, has found evidence of oxygen radicals killing cells alongside antibiotics in a variety of types of bacteria. In a 2009 study in Antimicrobial Agents and Chemotherapy led by microbiologist Xilin Zhao of Rutgers’ New Jersey Medical School, bacteria genetically engineered to lack metabolic enzymes that make hydrogen peroxide (a precursor to hydroxyl radicals) survived what should have been a lethal antibiotic attack. In 2012 in Science, Walker and colleagues published data suggesting that drug-derived reactive oxygen species damage the cell’s warehoused guanine bases — the G in the genetic code — furthering the central idea of Collins’ model.
The model has been particularly useful to tuberculosis researchers. In 2011, infectious disease researcher Harvey Rubin of the University of Pennsylvania and colleagues found evidence that clofazimine, a type of antibiotic that fights leprosy and drug-resistant tuberculosis, also sparks the formation of lethal reactive oxygen species in microbes. “There’s no question in my mind,” Rubin says. “In our hands, reactive oxygen species can be generated and can kill cells.” Rubin’s team published its results in the Journal of Biological Chemistry. In 2012, molecular biologist Deborah Hung of Harvard and her team built on the clofazimine data, reporting in the Proceedings of the National Academy of Sciences that the same damaging molecules could also annihilate lingering tuberculosis-causing microbes.
The conflicting data leave lingering questions about how antibiotics kill, says Wright. “There’s not a one-size-fits-all model.” But he’s reluctant to say that reactive oxygen species play no role. “I think that’s unlikely,” he adds. “What the role of reactive oxygen species is is going to be an interesting thing to figure out.”
Therapeutic fallout
Some worry that the backlash over Collins’ initial data has stalled efforts to make current antibiotics more effective. “The big deal is that we have squandered one of the most valuable medical advances of all time and we’re losing it,” Drlica says of antibiotic therapy. “If you don’t believe that Collins is fundamentally right, then you won’t pursue that line of investigation. That’s why it’s an important controversy.”
A handful of researchers, including Rubin and Walker, continue to explore the idea. In 2012, Collins cofounded EnBiotix with Wager, a venture capitalist. “Is there more to learn about how this mechanism works? Of course,” Wager says. “That doesn’t shake our confidence one iota regarding the validity of the general hypothesis.” EnBiotix aims to boost the amount of reactive oxygen species an antibiotic creates, thereby fortifying its killing ability, he says.
For Collins, continuing to study how current drugs kill is an obvious way forward. “We still have only scratched the surface of what’s happening with these drugs,” he says. Collins and his lab have written a paper, expected later this spring, that backs the reactive oxygen species model and addresses the weak points that Imlay’s paper pointed out. The debate isn’t over, he says. Yet more studies will be needed to completely understand how antibiotics kill and how to make them kill even better.
“Death,” he adds, “is a complicated process.”