A certain secret agent’s preference for martinis that are shaken — not stirred — might be all about the bonds. Scientists who have zoomed in on the molecular structure of several brands of vodka propose that differences in water-ethanol interactions may account for drink preferences.
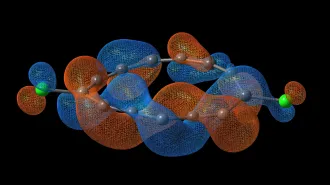
Some of vodka’s water molecules form cagelike structures around molecules of ethanol, a research team reports online May 21 in the Journal of Agricultural and Food Chemistry. Disrupting these cages — via impurities or perhaps even shaking — may affect taste, says study coauthor Dale W. Schaefer of the University of Cincinnati in Ohio.
Schaefer cautions that no data link such structural differences to brand preferences. But with more research, a measure of vodka’s microstructure could serve as an all-purpose quality control measure, he says.
Schaefer and colleagues from Cincinnati and Russia used spectrographic techniques to assess the liquid structure of five brands of vodka: Skyy, Belvedere, Stolichnaya, Grey Goose and Oval. Spectral readings of the vodka differed slightly from brand to brand, the researchers found, indicating structural differences in the water-ethanol solutions. The team attributed those differences to the proportion of ethanol molecules that are trapped in a cage of water. When ethanol and water mix, water becomes more structured and the bonds between oxygen and hydrogen tighten up, Schaefer says. Water’s hydrogen atoms are pulled closer to the oxygen atom, imparting a more rigid structure that allows the molecular cages to form, he says.
Belvedere and Oval appear to have more of this caged ethanol than other brands do, the team reports.
Other researchers aren’t sure that the water-molecule cages are actually present at ordinary temperatures. These crystallike cages, which can physically imprison other molecules, typically occur only at very low temperatures and high pressures, says theoretical chemist Joel Eaves of the University of Colorado at Boulder. (Such “clathrates,” as the cages are known, are responsible for the icy slush that clogged an attempt at capping the recent oil spill in the Gulf of Mexico.)
“These cages are very transient entities,” says Eaves. “It’s hard to see how relevant they are in terms of any of the [vodka] chemistry.”