- More than 2 years ago
Unlike blinged-out rap stars dripping with platinum chains, fuel cell designers try to scrimp on the precious metal. Researchers have now come up with a new way to make do with less platinum and get even better performance from fuel cells. The finding, which appears online May 14 in Science, may provide a much-needed price chop for clean, efficient fuel cell technology.
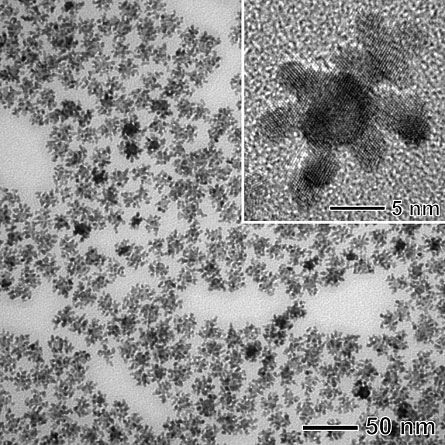
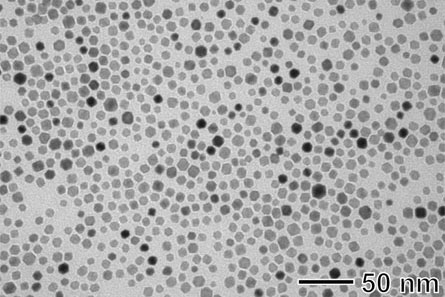
Fuel cells generate energy through chemical reactions between hydrogen and oxygen, emitting only water as waste. But the high cost of the materials needed to make these reactions happen — in particular, platinum — has prevented fuel cell technology from becoming prevalent. “The major issue is that reserves and depositions on Earth are very small,” says study coauthor Younan Xia, of Washington University in St. Louis.
Other metals such as lead and stainless steel have been tried as substitutes for the reaction-promoting material, called the catalyst, but they don’t perform as well. “So far, it’s no question. Platinum is the best in terms of performance,” Xia says.
Xia and his colleagues attempted to create an alternative catalyst by combining platinum and palladium, another metal (still precious, but not as rare as platinum). The researchers used a technique called “seeded growth,” in which they first made a base out of palladium molecules, which served as “seeds” for the platinum. Then, the researchers heated the material to about boiling temperature and added an early form of platinum, along with vitamin C. The reaction between the vitamin C and the platinum precursor made the platinum deposit in a pattern that looked like tiny fingers coming off a large palladium ball.
The high surface area of this formation makes the new material a highly efficient catalyst, while using less platinum, Xia says. The team reports that for every molecule converted per second by the commonly used, commercially available catalyst, two molecules are converted by the new material, doubling the activity. What’s more, the activity levels of the new catalyst almost meet performance targets for fuel cell catalysts set by the Department of Energy, an engineering feat.
“Overall, this is an exciting new development in controlled synthesis of unique nanostructures … for fuel cell applications,” says chemist Shouheng Sun of Brown University in Providence, R.I.
Durability, in addition to cost, has long been a problem for widespread fuel cell use, and the new material lasts only slightly longer than the commercially available catalyst, Sun notes. The researchers plan to spike their new material with a stabilizing agent made of gold, which is cheaper than platinum. “The authors clearly realized the problem,” Sun says. “Whether or not this will work waits to be seen.”






