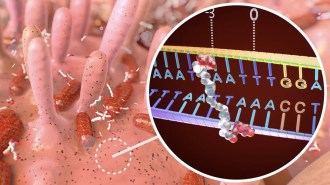
STARTING POINT Nearly 4 billion years ago, the chemical precursors to life may have all formed together in trickles of water and puddles on Earth's cracked and pummeled surface.
NASA's Goddard Space Flight Center Conceptual Image Lab
Scientists have long mulled over whether the protein or its genetic code came first. Or maybe it was the cell that houses them both. Now chemists may have the answer: The components for life all arrived together.
A series of primordial chemical reactions can generate the building blocks of the three necessary components for a living cell — genetic material, proteins and cellular compartments, researchers report online March 16 in Nature Chemistry. It’s the first experimental evidence that these chemical precursors to life could have arisen at the same place and time from the same ingredients.
That place and time could have been a network of streams and pools on Earth’s surface nearly 4 billion years ago, about the time when life started, the study’s authors suggest.
“It’s a chemical tour de force,” says chemist Terry Kee of the University of Leeds in England. The finding that the genesis of life’s components could have converged is an exciting step forward, says Kee, who was not involved in the study.
For decades, many chemists theorized that the genesis of cellular life’s components occurred in isolation, requiring different chemicals and conditions. But picturing the chemical origins of one aspect of life absent the others is like imagining the evolution of a human arm that’s been removed from the body, says chemist John Sutherland of the MRC Laboratory of Molecular Biology in Cambridge, England. “It looks really crazy,” he says.
Sutherland and colleagues built on their earlier discovery that two chemicals likely to be found on a lifeless Earth, hydrogen cyanide and hydrogen sulfide, could produce components of the genetic material RNA. Through a series of transformations involving electrons, carbon–containing molecules and exposure to ultraviolet light, the researchers generated two of the four building blocks of RNA: uracil and cytosine nucleotides.
Still using hydrogen cyanide and hydrogen sulfide, the researchers sprinkled in a few other ingredients, also likely to exist on a lifeless Earth, such as phosphates. The resulting chains of branched reactions, resembling a complex network, required occasional heating, irradiation and adding a new chemical or re-adding one of the two main chemicals.
“The key thing about the network is that although it looks complicated, it’s all the same reactions,” Sutherland says.
In addition to the two RNA molecules, the reactions led to 12 amino acids, the building blocks of proteins, and a precursor to lipids, the molecules that make up cellular membranes.
Sutherland and colleagues envision these reactions taking place in streams of water that trickled over the cracked landscape of a young Earth. There, the reactions would progress as the oozing water picked up molecules from the rocky terrain and meteorites that pummeled the planet. Occasionally, the streams may have partially evaporated and soaked up ultraviolet rays from the sun, spurring further reactions. Eventually, the dribbled chemicals could have pooled in primeval puddles where the first life assembled.
“This is one of the best, most complete and most thoughtful papers ever written about how the building blocks could have formed,” says chemist Doron Lancet of the Weizmann Institute of Science in Rehovot, Israel. But the next step, the assembly, is really the crux of the origins of life mystery, he says. “The paper lays a solid foundation of the first step so that we can free our minds to think about the second step.”