Catapults aren’t just handy for besieging medieval castles. They can also help battle invading bacteria, scientists have learned.
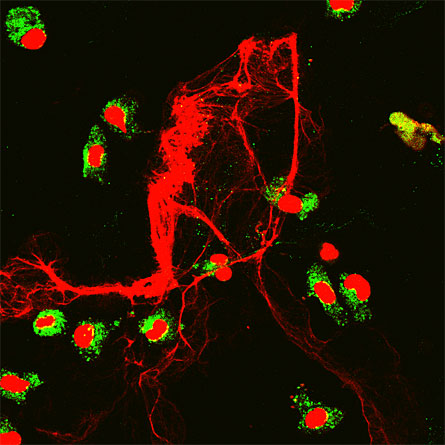
In the bacteria-laden gastrointestinal tract, a type of white blood cell catapults its own mitochondrial DNA out of the cell, creating a tangled trap of DNA and proteins that snares enemy bacteria. The research, published online August 10 in Nature Medicine, reveals a new role for this type of blood cell and suggests doctors should be cautious about drug treatments that target the cells.
“This is a really crazy finding,” says study leader Hans-Uwe Simon of the University of Bern in Switzerland. “The DNA was expelled within one second — and this is against tissue pressure and fluids, not air.”
Scientists already knew that the DNA-flinging cells, called eosinophils, secrete toxic protein granules during battles with foreign invaders. (The red dye eosin binds to these granules, hence the name.) Normal 0 false false false MicrosoftInternetExplorer4 Eosinophils make up only 1 to 3 percent of the body’s white blood cells and are found only in certain places, such as the digestive tract. The cells’ precise role in the immune system cavalry has puzzled researchers for a long time. Until recently, eosinophils were mostly studied in people with parasitic infections or asthma.
“Eosinophils are really the red-headed stepchild of white blood cells,” says study collaborator James Lee of the Mayo Clinic Arizona in Scottsdale. Immune system cells with more prominent roles, such as T cells, have garnered much more attention, Lee says.
“The med school paradigm is that eosinophils are a host defense against big parasites that can’t be gobbled up by smaller immune system cells. Instead you bring in these eosinophils that secrete a bunch of nasty things that kill them,” says Lee. From the body’s point of view, “This is a situational weapon, not the howitzer that you pull out to kill everything.”
When the eosinophils secrete their toxins, nearby tissues often experience collateral damage. The inflammation in some kinds of asthma results from eosinophils mistakenly recruited to the airway, where the cells’ toxins can be harmful.
The new work is exciting and makes a lot of sense, comments immunologist Paul Kubes of the University of Calgary in Canada. “When these cells are tackling some of the larger parasites, they release toxins that can float around and do damage,” Kubes says. But if the toxic proteins attach to DNA, there may be less random damage, he speculates. The DNA might prevent the proteins from moving about willy-nilly.
Simon’s team conducted several experiments that investigated eosinophil activities. The team took biopsies from the colons of people with Crohn’s disease, a disorder that involves inflammation of the digestive tract. In the diseased samples, the researchers identified expelled DNA, which wasn’t present in samples from healthy colons. The team also found expelled DNA bound to proteins in tissues from people with other bacterial and parasitic infections.
The team also exposed purified blood eosinophils to bacterial proteins for 20 minutes. Many of the cells released their DNA, the scientists report. Further experiments found that mutant mice that make bacterial protein had many more eosinophils than regular mice, and the mutants also recovered better from sepsis than regular mice
.
The scientists aren’t sure how the cells catapult their DNA with such force, Simon says. The mechanism may be similar to how some plants expel pollen. The team also speculates that the DNA-protein nets may actively lasso bacteria, or at least provide a physical barrier to the invasion.
The findings suggest that antiasthma drugs that target eosinophils should be used with caution, says Marc Rothenberg of the Cincinnati Children’s HospitalMedicalCenter in Ohio and director of the CincinnatiCenter for Eosinophilic Disorders. “Obliterating the body’s eosinophils may have consequences,” he says. “This is important work to highlight. It really brings forth a new hypothesis.”







