In a discovery that could have implications for biological research, new calculations show that a basic tenet of chemistry is wrong. At issue is why molecules twist into the shapes they do. The new finding indicates that in some molecules, quantum forces trump the traditional explanation.
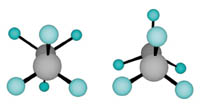
The find focuses on ethane, or C2H6, which has two carbon atoms bonded to each other. Each also attaches to three hydrogen atoms. On either end of the carbon-carbon bond, each hydrogen-adorned carbon atom, or methyl group, spins like a three-pronged turnstile, says chemist Frank Weinhold of the University of Wisconsin-Madison.
College instructors teach chemistry students to look at the molecule from one end, so that one carbon blocks the other from view. From this vantage point, students can distinguish the two ethane conformations. In the depiction of ethane’s stable, or so-called staggered, conformation, all six hydrogens are visible and equally spaced. In diagrams of the less stable, or eclipsed, conformation, the rear three hydrogens are hidden because they line up directly behind the front three hydrogens.
Textbooks give students a seemingly straightforward reason for the greater stability of the staggered form, says Lionel Goodman of Rutgers University in New Brunswick, N.J. They pin it on so-called steric effects. In the eclipsed conformation, close carbon-hydrogen bonds crowd each other, thereby raising the molecule’s overall energy.
But now, 50 years after this seductively simple explanation caught on, Goodman and his Rutgers colleague Vojislava Pophristic say that it’s not so. “The implications are rather large because the cornerstone of our understanding of the structure of molecules is the steric effect,” says Goodman. “And ethane is regarded as the benchmark for understanding the structure of molecules.”
Pophristic spent 5 years crunching calculations with a supercomputer to figure out what underlies the stability of ethane’s staggered conformation. First, she mathematically modeled ethane and removed the parts of the calculations that relate to the steric effect. To her surprise, the ethane molecule remained staggered. Something else must keep it in the staggered conformation.
Then, Pophristic looked at the other known influence on ethane’s twisting–a quantum mechanical effect known as hyperconjugation. “The electrons of one methyl group jump over to the other methyl group,” says Goodman.
When Pophristic blocked the electron jumping by placing a hypothetical screen between the two methyl groups, ethane’s structure finally assumed the eclipsed form, she and Goodman report in the May 31 Nature. Hyperconjugation, not steric effects, makes staggered ethane stable, they conclude. And what goes for ethane could go for many other organic compounds, says Goodman.
Researchers can no longer assume that steric effects play the major role in determining stable forms, comments Weinhold. They should give more attention to quantum mechanical effects when studying biological molecules and processes such as protein folding. “You eventually have to come to grips with this quantum mechanical aspect of everything,” says Weinhold.






