One day, during the spring semester of 1999, L. Bruce Railsback turned against one of science’s most visible icons: the periodic table of chemical elements. He was using a conventional periodic table mounted on the wall to illustrate a geochemistry lecture about the behavior of minerals in natural waters. That’s when he realized how confusing the table’s organization was, at least for his purposes. “I looked like a contortionist trying to point to different elements in different places,” says Railsback. “That’s what pushed me over the edge.”

To most people, the periodic table is the epitome of science at its most orderly. The table’s tidy rows and columns slot all of the 110 or so elements into fixed groups. However, to Railsback, an earth scientist at the University of Georgia in Athens, the table represents complete chaos.
“I thought, ‘There ought to be a way to group the elements that would make sense to someone interested in natural and geological processes,'” says Railsback. Back in his office, he set to work designing a new periodic table that would be more scientifically, and therefore more ergonomically, suited to an earth scientist.
Earth scientists encounter elements mainly in their ionic forms, where they carry a positive or a negative charge. So, Railsback organized his table around ions rather than the neutral atoms featured in the conventional periodic table.
“It’s a very nice tool, kind of like an expert system for viewing the periodic table in the context of a variety of applications,” says Laura Crossey, a geologist at the University of New Mexico in Albuquerque.
Railsback’s table is also emblematic of an ongoing quest among scientists to find the perfect format for the periodic table.
Labor of love
It took Railsback more than 4 years to produce a new chart–a project that culminated in the publication of “An earth scientist’s periodic table of the elements and their ions” in the September Geology.
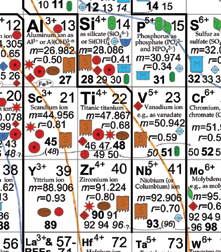
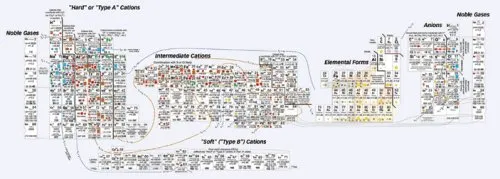
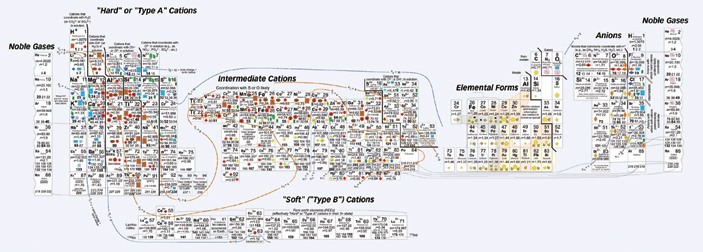
In this new version, Railsback changed the way the elements are grouped, although within those groups, he more or less maintained the order in which the atoms appear in the conventional table, that is, according to the number of protons in the atom’s nucleus. Blocks of elements make up six separate groups that are connected by colored lines. All the positively charged ions, or cations, are together on the right side of the collage, and all the negatively charged ions, or anions, are on the left. Uncharged elements constitute their own cluster, situated to the left of the table’s center. Two copies of the column with the noble gases, such as helium and neon, bracket the table like bookends.
Many elements appear in the table more than once. Sulfur, for instance, appears four times–S, S2+, S4+, and S6+. “It reflects the different ways sulfur can behave in nature,” says Railsback, adding that sulfur is found in dissolved forms in the ocean as well as in various solid mineral forms.
In his chart, a colored shape within the box for each element indicates where in the environment a particular ion is concentrated–in Earth’s mantle and crust, soils, seawater and rivers, the atmosphere, or living organisms.
Elaborate contour lines add another dimension to the table. Much as markings delineate a topographic map, a series of lines meander around groups of ions. These lines demark similar ionic potential, which is a measure of how tightly an ion’s charge is packed. The larger the potential, the more the ion will attract particles of opposite charge or repel particles of similar charge.
“That’s going to have a lot to do with how that ion behaves in solution,” says Railsback. A positively charged ion with a high ionic potential will react more readily with the negatively charged ends of water molecules than will a cation with a smaller ionic potential.
Because ions with similar ionic potentials tend to concentrate in the same sorts of environments, the contour lines link groups of elements with same colored symbols. The overall effect: Speckles of different colors cluster in swaths across the table. A swath of blue represents ions in seawater, and a band of brown represents ions in soils.
“It’s a fabulous piece of work,” says Richard Wanty, a research chemist at the United States Geological Survey in Denver. Wanty was particularly pleased to see his favorite element, vanadium, appear three times–in the ionic forms V3+, V4+, and V5+. “In the normal periodic table, you get one shot at vanadium and that’s it,” he laments.
From the behavior of gold in mineral deposits to the uptake of potassium ions by plants, “there’s something for everybody in this periodic chart,” says Wanty.
For example, a scientist specializing in dating ancient rocks might want to investigate potential problems in using the decay rates of radioactive uranium to determine the age of materials (SN: 9/6/03, p. 147: Art on the Rocks: Dating ancient paintings in the caves of Borneo).
Railsback’s chart shows that the uranium ion, with a charge of +6, falls among materials with very high ionic potentials and therefore high solubilities in water. There’s plenty of water in geologic settings, such as limestone caves, so uranium will leach away.
Says Railsback: “If we start losing material, then our data is no longer correct.”
The new ionic organization of elements could also be useful for materials scientists, Crossey suggests. In recent years, researchers have made great strides in fabricating a wide range of biomaterials that interact with ions. Examples include materials made from proteins and synthetic polymers that can remove mercury ions from water, and polymers that release drugs in the body when concentrations of calcium or magnesium ions are high enough (SN: 3/8/03, p. 150: Available to subscribers at Making Polymers That Self-Destruct: Layers break apart in controlled way).
Now that the table is publicly available, Railsback hopes that researchers and educators will take note. Wanty, for one, is an early convert. “If I were to teach a geochemistry class, I would start here,” he says.
Table manners
Railsback isn’t the only one inclined to modify the conventional periodic table into a more specialized and revealing reference suited to his specialty.
In fact, there is a long tradition of modifying the chart among geologists, metallurgists, and even biologists, says Eric Scerri at the University of California, Los Angeles. “People in different disciplines will want different things out of the periodic table,” says Scerri, who specializes in the historical and philosophical aspects of the periodic system of elements. Keeping the order of the elements fixed, the table can be molded in any number of ways. “It’s infinitely flexible. . . .
That is one of the strengths of the periodic table,” he says.
From spirals and trees to three-dimensional pyramids, numerous alternative tables have been proposed.
While many of these tables bring the conventional table to life, some are downright wacky, says William Jensen, a chemistry historian at the University of Cincinnati. Last year, he notes by way of an example, a high school teacher drove across the country to show Jensen his own version of the table. “He had a 1957 Cadillac, and on it was painted the periodic table,” says Jensen.
The Internet, with its hundreds of Web sites dedicated to the periodic table, has added a hypertextual dimension. Through links and interactive features, users can retrieve a wealth of data on the chemical and physical properties of different elements, including animated visuals of an element’s orbiting electrons and a brief history of each element’s discovery.
Despite many creative variations, the fundamental organizing principle of the periodic table has remained largely unchallenged for more than 130 years. It’s survived a number of scientific revolutions such as quantum mechanics.
The table was devised in 1869 by the Russian chemist Dimitri Ivanovich Mendeleev. His tack was to order the elements on the basis of their atomic weights–that is, the number of protons and neutrons in an atom’s nucleus. Moreover, he arranged the elements in columns on the basis of the number of chemical bonds an atom can form.
Early in the 20th century, one of these fundamental rules for the table changed slightly. Elements have since been ordered according to their atomic numbers, or number of protons.
The last major change to the table was in the 1940s, when Nobel Laureate Glenn Seaborg at the University of California, Berkeley created a separate group for the lanthanides and actinides–the rare earth and radioactive elements.
Before Seaborg’s revision, these elements were mixed in with the transition metals in the middle of the chart. While bombarding uranium with neutrons in the lab, Seaborg discovered that the actinides shared their chemistry primarily with the lanthanides. Therefore, he concluded, the two types of elements warrant their own block.
“That resulted in a very fundamental change in the whole structure of the periodic table,” says Jensen.
All subsequent changes to the conventional table have revolved primarily around esthetic issues, says Jensen. The questions tend to be, “Do we want the table in the shape of a cone or a fan?” he says.
“But all these things are really devoid of any real scientific content.
They are not really telling us anything new or insightful about the nature of the elements.”
Stirring the beast
Most scientists agree that the order of atoms in the table will never change, unless, of course, a major revolution turns upside down everything known today about matter. Even so, many researchers contend that the current organization of the table is far from perfect.
“Should the form of the table be the conventional form, which is rather ugly and asymmetrical, or could it be more symmetrical?” asks Scerri. This is only partly a matter of aesthetics.
Scerri and other chemists have begun scrutinizing the placement of helium at the top of the right-hand column in the conventional table.
That’s the column housing the noble gases, so-called because of their penchant for not engaging with other elements. What makes helium odd among the noble gases is that instead of having six electrons in its outer subshell, it has only two electrons. This suggests, says Scerri, that helium belongs on the far left of the chart with the alkaline earth metals, which also have two electrons in their outer shells.
“Chemists are a bit horrified by the idea of putting helium in with a bunch of metals,” says Scerri. But he argues that placing helium with the alkaline earth metals results in a more regularly shaped structure that can take two forms. One form, referred to as a pyramid, is a stack of blocks with increasing length from top to bottom. The other resembles a set of stairs descending to the left. Helium sits next to hydrogen at the top of the stairs. This left-step table places atoms in different rows on the basis of the size and shape of an atom’s electron orbitals.
Other scientists have proposed this particular structure before, Scerri notes, but the idea has been gaining momentum in recent years. “Now, something might actually happen,” says Scerri.
Getting the entire world to adopt the same, new periodic table will be a challenge, especially because the table will need to garner approval from the International Union of Pure and Applied Chemistry.
Traditionally, chemists have not taken well to attempts to change the table. “I call this ‘the tyranny of the chemist,'” says Scerri.
In the meantime, researchers like Railsback are less concerned with the wholesale adoption of a new table than molding the table to suit their needs. As more scientists pursue that end, the periodic table will continue to evolve into a diversity of forms, each one filling a niche.
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.