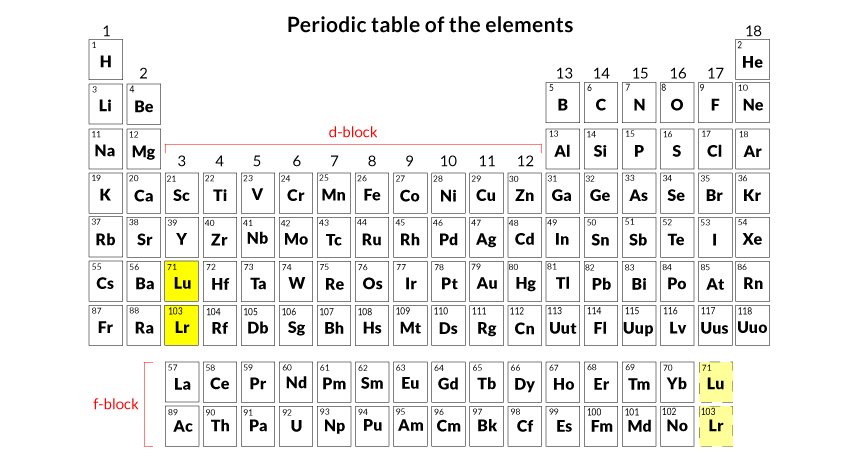
FLEETING Despite new results, controversy remains over where the radioactive element lawrencium (plus its upstairs neighbor lutetium) should be in the periodic table: in the d-block or f-block.
E. Otwell

FLEETING Despite new results, controversy remains over where the radioactive element lawrencium (plus its upstairs neighbor lutetium) should be in the periodic table: in the d-block or f-block.
E. Otwell