
CHEMICAL BLOCKBUSTER Film of a fiery reaction explains the surprising trigger for a classic explosion.
Phil Mason
Lights, camera, kaboom! With snapshots from a high-speed camera, chemists can finally explain why sodium and other alkali metals blow up in water.
Just before the explosion, spikes burst from the metal’s smooth surface, setting off a chain reaction that ignites the metal. The blast’s film debut, appearing online January 26 in Nature Chemistry, offers a long-awaited explanation of a classic chemical reaction demonstrated in classrooms worldwide.
“What we found out is that there’s a crucial piece of the puzzle that precedes the explosion,” says computational chemist Pavel Jungwirth of the Academy of Sciences of the Czech Republic in Prague.
In textbooks, chemists describe the reaction in simple terms. Alkali metals, a group of elements including potassium and sodium, are highly reactive. In a splash of water, the metal jettisons electrons, which generates heat. Once afloat, these liberated electrons attack water molecules, breaking off hydrogen atoms to form explosive hydrogen gas. The gas then ignites in that newly-generated heat.
In order for electrons to jump ship, the metal and water have to be in direct contact. But Mason theorized that before enough heat could build up to ignite the hydrogen gas, the extra warmth would also create steam from the surrounding water. A blanket of vapor on the metal would block fleeing electrons and halt the heat-up. The chemical reaction, Mason reasoned, should smother itself.
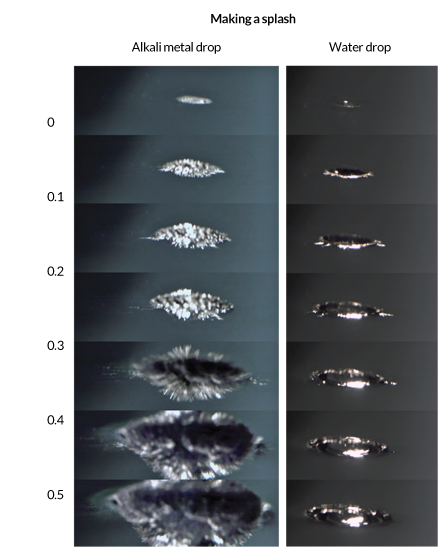
Mason and Jungwirth decided to film the explosion of an alloy of sodium and potassium. A liquid at room temperature, this blend of alkali metals offers a predictable blast, scene after scene — unlike pure alkali metals, which can produce inexplicably varied explosions. To catch the fiery events, the researchers started with a cheap camera that takes 500 images per second before turning to a camera capable of snapping 30,000 images per second.
With a camera rolling, the researchers held a 100 milligram glob of the alloy a meter above a pool of water, then let it drop. At 0.5 milliseconds after the metal touched the water, the explosion was in full swing. But just before that — at 0.35 milliseconds — something weird happened: the metal’s smooth surface became spiky, like an angry hedgehog. This detail gave the chemists the answer they were looking for.
The spikes that shoot from the surface are pieces of positively charged metal, the chemists say. Once electrons abandon the surface of the metal, they leave behind positively charged atoms, which repel each other and create spikes as they leap away. This creates gaps in the surface, exposing underlying atoms to the water. These newly exposed atoms then lose electrons, creating more positive atoms that form spikes upon spikes. As the metal continually unloads electrons, the heat needed to ignite the hydrogen gas builds up before steam can stifle the explosion.
Computer simulations of the reaction backed up the chemists’ explanation.
“It makes sense,” says organic chemist Rick Sachleben of Momenta Pharmaceuticals in Cambridge, Mass.
Like Jungwirth, Sachleben hopes that the new finding makes its way into chemistry classrooms in addition to laboratories. It’s a perfect example of taking a long-held assumption, questioning it, and coming up with a better understanding, Sachleben says. “It could be a real teaching moment.”
SHARP BLAST Just before it ignites in water, an alkali metal (Na/K, an alloy of sodium and potassium) loses electrons and shoots spikes from its surface, which can be seen at the top of this slow-motion footage. A water droplet’s dive can be seen for comparison at the bottom. Credit: P. Mason et al.






