A cancer drug that sweeps an ominous plaque-forming protein from mouse brains within hours and reverses Alzheimer’s-like behavior in the rodents in days may offer a powerful new way to prevent or even reverse the brain-wasting disease in humans.
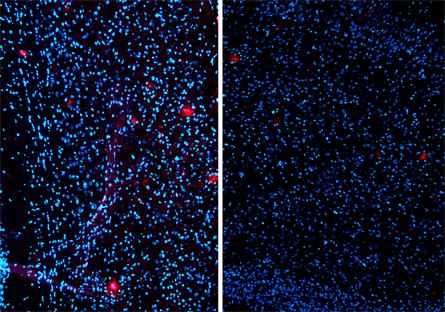
The drug, called bexarotene, has been on the market for about 10 years to treat T-cell lymphoma, often after other treatments have failed. But bexarotene can do a completely different job in the brain, researchers report online February 9 in Science.
“This is a pretty fantastic drug,” says Paige Cramer of Case Western Reserve University School of Medicine in Cleveland, coauthor of the new study, which used bexarotene to treat mice suffering from an Alzheimer’s-like condition.
The brains of people with Alzheimer’s disease are marked by high levels of a protein called amyloid-beta that can exist in both small fragments that scramble nerve cell communication and in large, sticky clumps called plaques.
Cramer and her colleagues studied mice that had brains full of both kinds of A-beta. Just hours after mice began taking bexarotene, levels of the smaller kind of A-beta in their brains fell, reaching a 25 percent reduction after 24 hours. After 14 days of treatment, plaque levels fell by 75 percent, the team reports.
“Nothing tested comes anywhere close to the speed with which existing amyloid is washed away by this drug,” says neurologist and neuroscientist Samuel Gandy of Mount Sinai Hospital in New York City.
The drug works by changing the behavior of a cholesterol-carrying protein called ApoE by targeting a protein that regulates it. (ApoE is no stranger to Alzheimer’s researchers: People with a particular version of ApoE have a higher risk of the disease.) Earlier studies have found that ApoE shuttles A-beta out of the brain, acting, as coauthor Gary Landreth of Case Western calls it, as “a garbage disposal.” By improving ApoE’s ability to remove A-beta, “bexarotene helps Mother Nature do what she normally does,” Landreth says.
Clearing out the A-beta from the brain had a profound effect on the mice’s behavior, too. Mice loaded down with A-beta have memory deficits and can’t learn new things as well as normal mice. After taking bexarotene for several days, animals showed a startling improvement in several different kinds of tests.
Normally, mice that are put into cages with a pile of soft tissue paper begin to chew the paper and form it into a soft pile, making a nest. The mice with an Alzheimer’s-like condition lost the ability to make the association between seeing the paper and the possibility of a soft place to sleep. But after three days of taking bexarotene, these mice began making nests again, the team reports.
Another measure of performance came from testing the sense of smell, which people often lose in the early stages of Alzheimer’s disease. When mice smell a strong odor repeatedly, they get used to the odor and don’t react strongly the third, fourth or fifth time they smell it.
Mice with brains full of A-beta didn’t get used to the odor, though, acting surprised each time they encountered the scent. But after treatment with the drug, the mice recovered the ability to get used to an odor. In these and other tests, abnormal behavior was reversed in mice that had received bexarotene.
More work is required, such as replicating the study with the human version of ApoE (the researchers used the mouse version of the protein). But since the drug is already FDA-approved, says neuroscientist David Holtzman of the Washington University School of Medicine in St. Louis, “you have a very clear path forward in humans.”
Landreth and Cramer hold a provisional patent application on using bexarotene as an Alzheimer’s therapy, and have started a company called ReXceptor Inc. to conduct more research. The team plans to test bexarotene in people in the coming months.
“Making the leap from mice to humans is the most difficult step in drug development,” says Gandy. “Based just on odds, I would bet against the drug, but the mechanism is novel and appealing, so I’m hoping that it beats the odds.”
In addition to its promise in treating Alzheimer’s disease, bexarotene might offer scientists a way to clarify A-beta’s role in the disease. The relationship has gone untested because scientists haven’t had a good way to reduce A-beta. “This is a perfect tool for testing the amyloid hypothesis,” Gandy says.
Back Story – SCRAPING BY
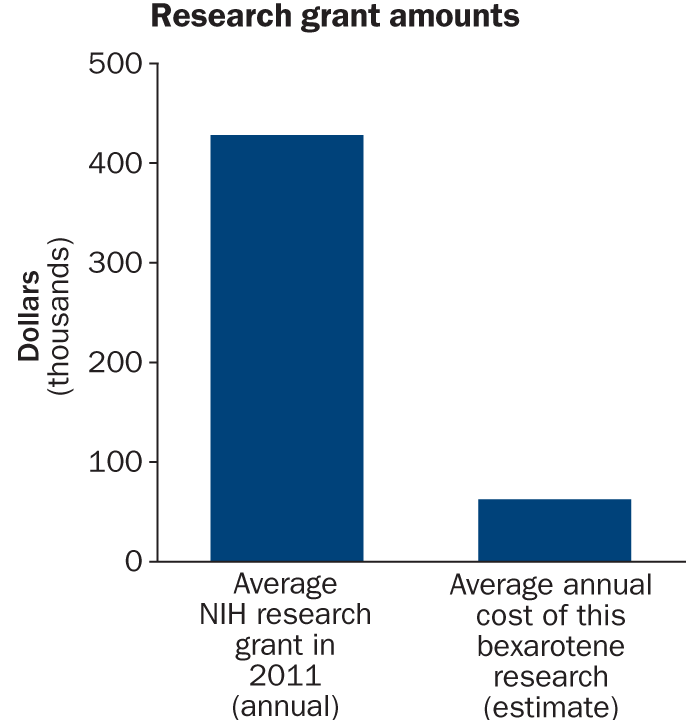
Source: NIH
Biomedical advances often come from large-scale projects supported by million-dollar grants. Yet Gary Landreth and his graduate student Paige Cramer conducted their study of the effects of bexarotene on Alzheimer’s-like dementia in mice on a budget of $250,000 spread out over four years. That’s less than a typical single year of support from many funding agencies.
The shoestring budget stemmed in part from the risky nature of the study. Bexarotene was predicted to have a “boatload” of side effects because its target partners with lots of other proteins, Landreth says. “No one in their right mind would have funded this thing initially.”
But with a small amount of no-strings-attached cash from the Blanchette Hooker Rockefeller Foundation, Landreth and Cramer were able to get initial results that looked promising enough to land a $200,000 grant from the National Institutes of Health. The pair quickly ran through that money, but not before they had found that bexarotene seems to ease symptoms of Alzheimer’s in mice.