Old periodic table could resolve today’s element placement dispute
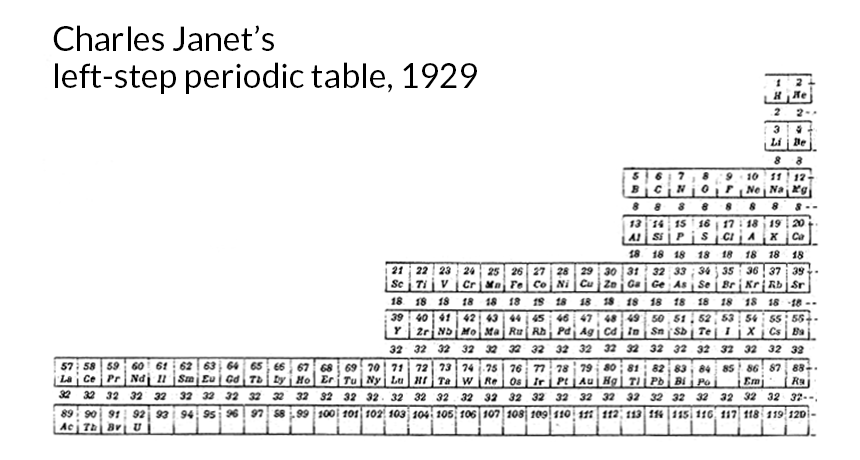
In the late 1920s, the French amateur scientist Charles Janet devised various versions of the periodic table of the chemical elements, basing the positions of the elements on patterns in the arrangement of electrons around the atomic nucleus. His "left-step" form included the proper positions for many elements discovered or created in the lab decades later.
If you ever want to open a chemistry theme restaurant, you should be sure to furnish it with 118 tables — one for each element. Note that it could not be a Greek restaurant, because then the number of tables would be limited to four. Yours, instead, would be a geek restaurant. You could call it The Periodic Tables.
Anybody who has ever had an encounter with chemistry should get the joke. The periodic table of the chemical elements is found decorating the walls of chemistry classrooms everywhere. It sums up the foundations of chemistry in a single convenient chart. There’s one square for each element, giving its name or symbol, atomic number and usually atomic weight as well.
In the usual display, each column of the table includes elements with similar properties. That’s a way of showing that certain properties recur at regular intervals, or periods, as you list the elements in order by their atomic number.
But the usual depiction is not the only way to organize the periodic table. In fact, hundreds of versions have been constructed since the first one, devised by the Russian chemist Dmitrii Mendeleyev in 1869. And those versions have occasionally disagreed about where some of the elements should be placed. Most such disputes were resolved long ago, but one stubbornly persists, as Science News chemistry writer Beth Mole recently reported (also noted previously here by Davide Castelvecchi of Nature).
In this case, the element in question is lawrencium, number 103 (along with the element that sits above it, lutetium, number 71). Typically you’ll find lawrencium at the bottom right, the last element in the actinides, relegated to the bottom of two rows appended under the main body of the table. But in fact (to tip my hand about how I think this dispute should be settled), lawrencium should actually occupy the spot in the main table between elements 88 and 104.
Curiously, the basis for this position was established many decades ago, but widely ignored by chemists and physicists and textbook publishers, probably because they had never heard of the person who figured it all out. He was Charles Janet, a French amateur scientist. Janet was born in Paris in 1849, went to engineering school and eventually joined his father-in-law’s business (a brush factory) and became wealthy. His leisurely pursuits included collecting fossils and publishing scientific papers about them as well as on other topics such as geology, plants and especially social insects.
First published in 1928, Janet’s table correctly positioned the actinide elements, many years before most of them were even discovered or created in the lab. And Janet’s table also correctly located elements 104 to 118, several decades before they were discovered.
In the late 1920s, Janet turned his perceptive powers to the chemical elements. He designed various new versions of the periodic table. Unlike most other tables constructed before then, his actually reflected the underlying reason why the periodic table works so well: the regularities in the arrangements of electrons surrounding an atom’s nucleus.
When Mendeleyev built his table (just before the German chemist Lothar Meyer developed a very similar version independently), nobody knew much about how atoms were put together. Some scientists still believed, as the ancient Greek atomists had, that an atom was an indivisible piece of matter with no internal parts. But astute observers realized that atoms behaved chemically in ways that suggested complex structures. Way back in 1815, the English physician William Prout hypothesized that all atoms consisted of conglomerations of a certain number of the simplest atom, hydrogen. Each atom’s weight, in that case, would reflect how many hydrogens it contained. By Mendeleyev’s time, atomic weights mostly appeared not to be whole numbers, though. Still, by ordering the elements from lightest atomic weight to heaviest, Mendeleyev discovered the patterns of repeating properties that his table displayed so well.
By the opening of the 20th century, the existence of the electron and the phenomenon of radioactivity showed that atoms contained electrically charged parts. After 1911, Ernest Rutherford’s discovery of the atomic nucleus and then Niels Bohr’s quantum theory of the atom of 1913 established that electrons swirled around a very small and dense nucleus. Soon thereafter, H.G.J. Moseley established that the amount of positive charge contained in each nucleus (its atomic number — the number of protons) provided the correct order for positioning the elements in the periodic table. (Atomic weight came close, but it was really the atomic number that mattered.)
As Bohr perceived, the atomic number mattered because it determined the number of an atom’s electrons, and the electrons governed the atomic interactions that determined the chemical properties. In the early 1920s, Bohr attempted to discern the electronic arrangement (or configuration) for the elements based on spectra — the different frequencies of light emitted by various atoms when properly disturbed. Bohr surmised that electrons arranged themselves in “shells” surrounding the nucleus. Tightly bound electrons, with relatively low energy, occupied shells near the nucleus. More energetic electrons traveled in bigger shells, farther away. Being bigger, the more distant shells could contain more electrons.
Within the larger shells, Bohr realized, electrons could occupy “subshells” corresponding to elliptical orbits. He described main shells and subshells with quantum numbers related to an electron’s energy and angular momentum. (Nowadays, of course, the idea of electrons “orbiting” within “shells” has been superseded by the fuzzier notion of occupying “orbitals,” regions of space in which an electron is likely to be found.)
Spectra revealed that electrons arranged themselves in patterns related to the quantum numbers. Bohr noted that recurring patterns in those arrangements were responsible for the periodicity of chemical properties that the periodic table depicted. In 1922 he produced a version of the table (modified from one developed by the Danish chemist Julius Thomsen in 1895), based on electron configurations. Good spectra were not available for all elements, though, and the proper positioning of some remained uncertain.
When Janet came along a few years later, he was aware of Bohr’s work. But Janet took a different approach, not so much using spectra to figure out electron configurations, but using mathematical patterns in the numbering of the shells to figure out what the electronic configuration ought to be. As electrons are added to an atom (that is, as atomic number rises), Janet realized, they arrange themselves to achieve energetic stability. In other words, if a lower energy state is available, an electron will fall into it; additional electrons fill shells of higher energy. That order of shell-filling depends on both the main quantum shell number and the subshell number. In today’s terminology, the subshells are orbitals, designated by letters s, p, d or f. The first energy level has one s orbital; the second energy level has one s and three p orbitals; the third has one s, 3 p’s, and five d’s; the fourth has, in addition to those, seven f orbitals.
By sometime in 1926, the German physicist Erwin Madelung had figured out a convenient rule for how electrons arrange themselves. It depends on the sum of the main energy level’s number and the orbital’s number (s=0, p=1, d= 2, and so on). Shell-orbital combinations with the lowest sum get filled first. So 4s, for instance (4+0=4) gets filled before 3d (3+2=5). If the sums are the same (4p=5 and 3d=5, for instance) the one with the lower main number gets filled first. Madelung didn’t publish this insight until a decade later. But in the meantime, Janet somehow figured it out for himself. His table gave the proper positions for all the elements, based on the periodicity in electron configurations dictated by Madelung’s rule. First published in 1928, Janet’s table correctly positioned the actinide elements (from 89, actinium, to 102, nobelium), many years before most of them were even discovered or created in the lab. (Unaware of Janet, chemists had originally mispositioned the actinides along the bottom row of the main part of the table.) And Janet’s table also correctly located elements 104 to 118, several decades before they were discovered.
Janet constructed several versions of his table. The one showing the positions most clearly (the “left-step” version) places element 103 (lawrencium) and lutetium directly below scandium and yttrium, showing that they belong in the main part of today’s table, not in the appended rows below it.
But Janet’s work was largely ignored. French wasn’t the best language for publishing chemistry papers in those days, and he was not known among the professional communities of chemists or physicists. Plus, as my Science News colleague Lila Guterman points out, his table did not fit neatly on a single sheet of paper.
It’s too bad nobody paid attention, because Janet had a lot to offer. “He was a man of astonishing originality,” ecologist Philip Stewart wrote in 2010 in the journal Foundations of Chemistry.
For one thing, Janet suggested the existence of an unknown element zero, and therefore the possibility of a “mirror” periodic table consisting of elements with negative atomic numbers — in essence, a prediction of the existence of antimatter. He also alluded to other phenomena that sound suspiciously like later discoveries, such as heavy hydrogen, nuclear fusion and helium-3, Stewart pointed out.
And by the way, Janet’s table did not stop at element 118, the highest atomic number atom now known, but also properly positioned the next two numbers. So when scientists succeed at creating number 119, they’ll know just where in the periodic table it should go. And they should name it Janetium.
Follow me on Twitter: @tom_siegfried







